Protoporphyrin IX Plays a Vital Role in Biological Systems
Life on earth is critically dependent on the existence of certain key molecules; among these critical molecules are a group of compounds containing tetra-pyrrole macrocycles as an important part of them.
Heme (Iron protoporphyrin IX), chlorophyll, and cobalamin (vitamin B12) are some of the tetra-pyrrole compounds vital to the survival of life — all of which require protoporphyrin IX to exist. Iron protoporphyrin IX plays a pivotal role in oxygen transportation when in the form of hemoglobin and is an active component of biologically essential molecules such as cytochrome p450, myoglobin, and numerous other metalloenzymes.1
1. Mense, S., Zhang, L. Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res 16, 681–692 (2006). https://doi.org/10.1038/sj.cr.7310086
What is Protoporphyrin IX?
Protoporphyrin IX (also referred to as Protoporphyrin or PPIX) is the macrocyclic ring system of heme without the iron chelated into the center of the molecule. The enzyme ferrochelatase is responsible for facilitating the chelation of iron into the center of protoporphyrin to form heme. Interestingly, the aromatic porphyrin ring system in protoporphyrin IX strongly absorbs light in specific regions of the visible spectrum of light giving blood its characteristic red color. Of its many important roles, perhaps most notable is that protoporphyrin IX is a critical intermediate in the biosynthesis of heme, chlorophyll, and cobalamin.
Did you know?
Protoporphyrin IX’s chemical structure was first proposed by Kuster in 1912 and was confirmed when Fischer reported the chemical synthesis of heme in 1929.
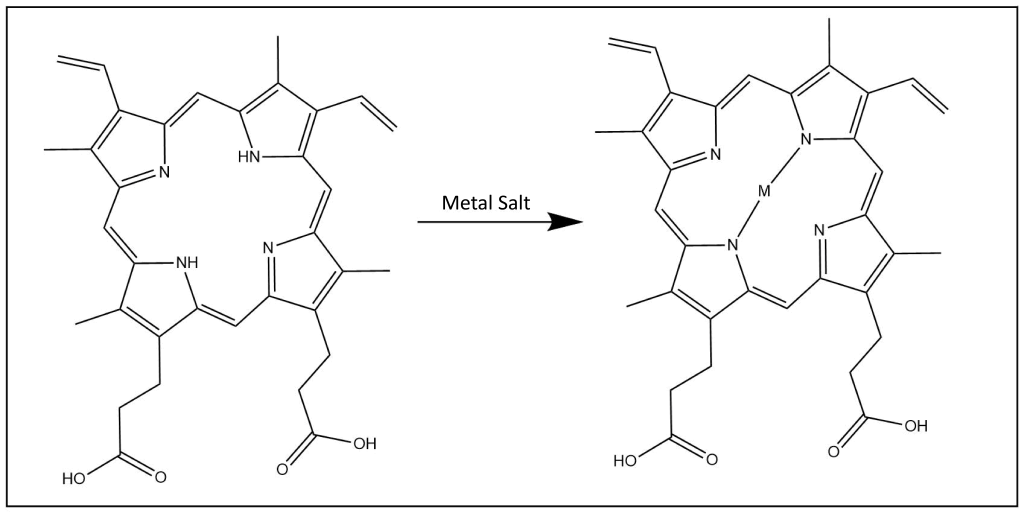
Protoporphyrin IX contains functional groups (carboxylic acids and vinyl groups) that have been modified to generate many unique compounds of interest. The free base form of protoporphyrin IX has been metalated with a variety of metals other than iron, imparting unique properties that can be used to research biological mechanisms and may impact the activity of enzymes in various biological pathways. The introduction of metalo-protoporphyrins using metals that don’t occur in nature have been used to explore a multitude of applications such as photosensitizers used in photodynamic therapy (PDT) to treat cancer, catalysts in organic transformations, and as a unique antimicrobial agent.
It is protoporphyrin IX’s extensive customizability that gives it such a unique and impactful role in various fields of research and application. You can see how vastly it can be customized by checking out our exhaustive (but not comprehensive!) list of custom porphyrins that we offer.
The Biosynthesis of Protoporphyrin IX for Hemoglobin, Chlorophyll, and Cobalamin (Vitamin B12)
The biosynthetic pathway to produce protoporphyrin IX represents one of the most essential metabolic pathways in living organisms, serving as a critical juncture in cellular metabolism. Protoporphyrin IX is the key precursor for the biosynthesis of several vital biomolecules, including hemoglobin, chlorophyll, and cobalamin (vitamin B12).
Hemoglobin, an essential protein found in red blood cells, is responsible for oxygen transport throughout the body. The incorporation of iron into protoporphyrin IX forms heme, the functional component of hemoglobin, enabling efficient oxygen binding and release.
In plants and certain algae, protoporphyrin IX is the precursor for chlorophyll, the green pigment crucial for photosynthesis. Chlorophyll’s role in capturing light energy and converting it into chemical energy through the photosynthetic process is fundamental to the energy balance of our planet.
Additionally, protoporphyrin IX serves as a precursor in the synthesis of cobalamin, commonly known as vitamin B12. Cobalamin is an enzymatic cofactor essential for both mammals and bacteria. This vitamin is vital for various metabolic functions, including DNA synthesis, fatty acid metabolism, and neurological function.
Thus, the pathway leading to the production of protoporphyrin IX is indispensable for sustaining life, influencing a wide array of physiological processes across different biological kingdoms.
Protoporphyrin IX is synthesized in 7 steps
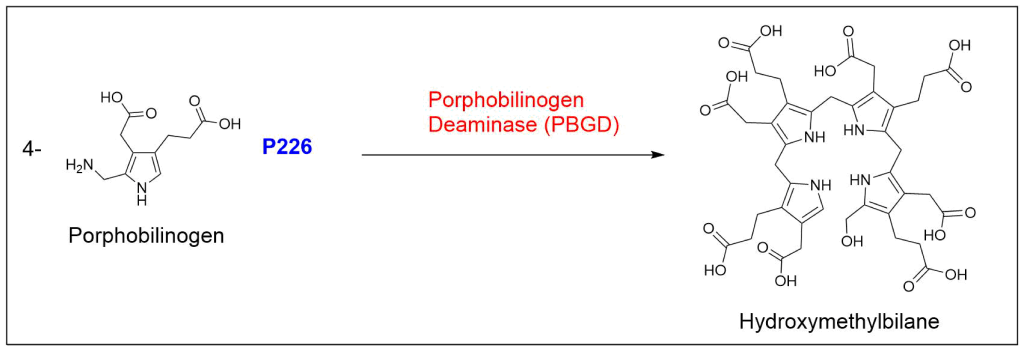
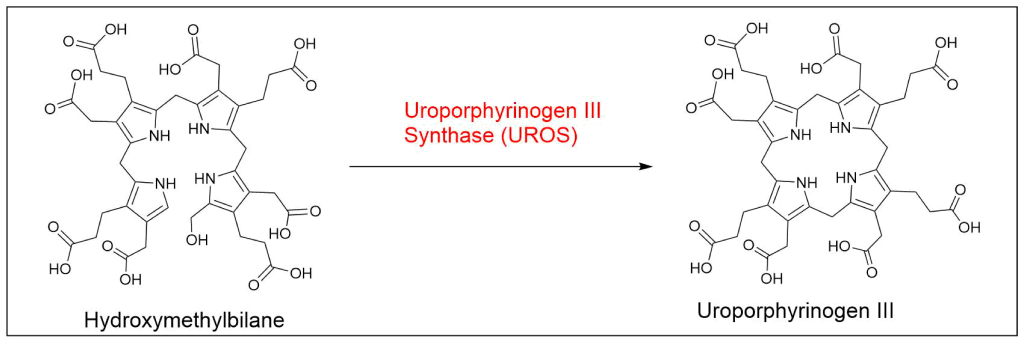
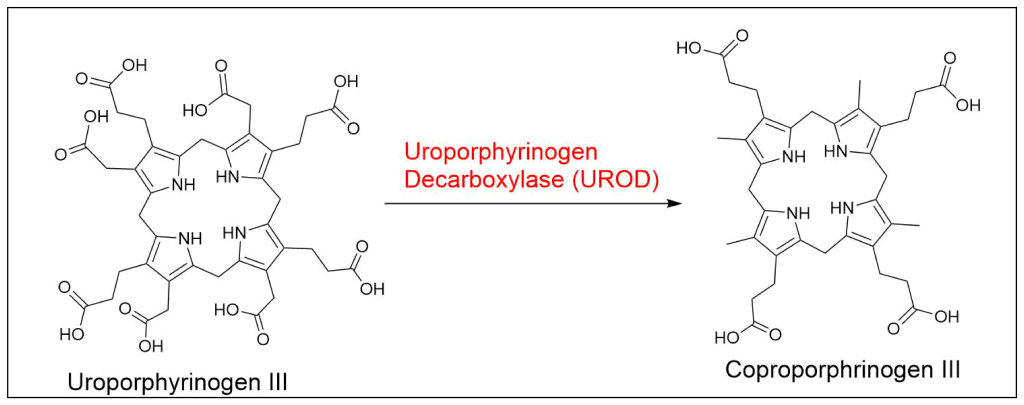
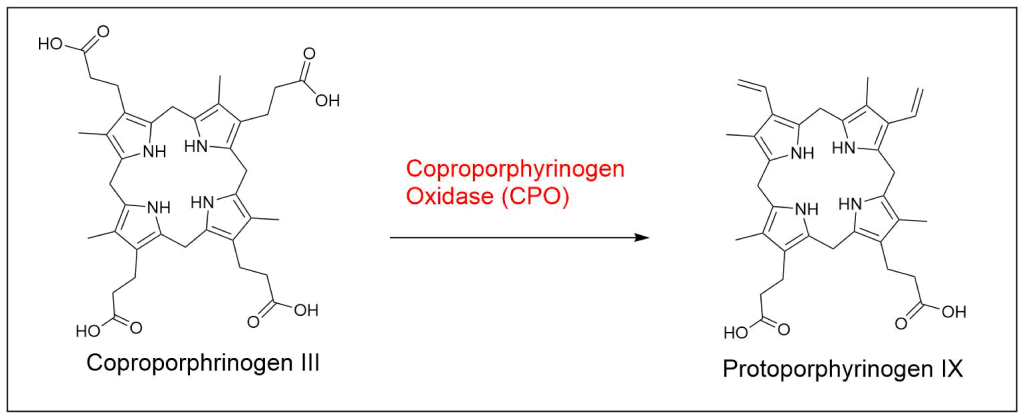
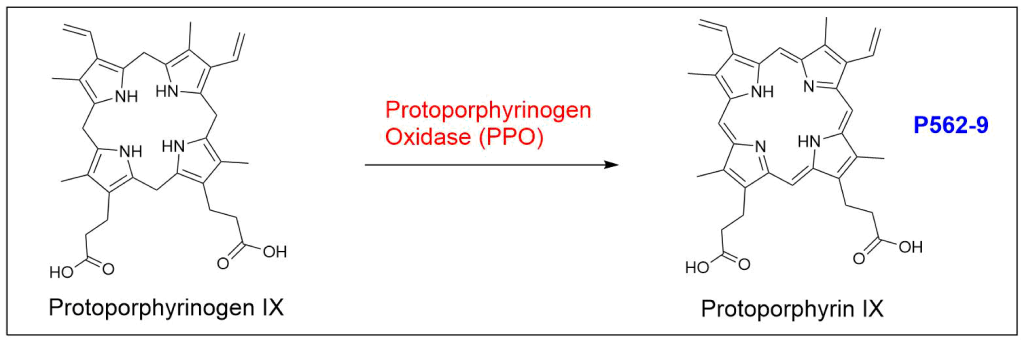
At last, the formation of protoporphyrin IX marks the final unifying phase in the biosynthesis of heme, chlorophyll, and cobalamin; from this point onward, the synthesis pathways diverge, each leading to the formation of these distinct but equally crucial biomolecules.
How is protoporphyrin IX used in further biosynthetic pathways?
The biosynthesis of heme only requires 1 additional step…
The biosynthesis of chlorophyll continues with 7 more steps…
The biosynthesis of cobalamin requires 17 steps of additional transformations…
- Ferreira, G.C., Gong, J. 5-Aminolevulinate synthase and the first step of heme biosynthesis. J Bioenerg Biomembr 27, 151–159 (1995). https://doi.org/10.1007/BF02110030
- Beale SI, Castelfranco PA. 1973. 14C incorporation from exogenous compounds into delta-aminolevulinic acid by greening cucumber cotyledons. Biochem Biophys Res Commun 52:143–149. https://doi.org/ 10.1016/0006-291X(73)90966-2.
- Beale SI, Gough SP, Granick S. 1975. Biosynthesis of delta-aminolevulinicacid from the intact carbon skeleton of glutamic acid in greening barley. Proc Natl Acad Sci U S A 72:2719–2723. https://doi.org/10.1073/ pnas.72.7.2719.
- Huang DD, Wang WY, Gough SP, Kannangara CG. 1984. Deltaaminolevulinic acid synthesizing enzymes need an RNA moiety for activity. Science 225:1482–1484. https://doi.org/10.1126/science .6206568.
- Kannangara CG, Gough SP. 1978. Biosynthesis of delta-aminolevulinate in greening barley leaves: glutamate-1-semialdehyde aminotransferase. Carlsberg Res Commun 43:185–194. https://doi.org/10.1007/ BF02914241.
- Schon A, Krupp G, Gough S, Berry-Lowe S, Kannangara CG, Soll D. 1986. The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature 322:281–284. https://doi.org/ 10.1038/322281a0.
- Jaffe, E.K. Porphobilinogen synthase, the first source of Heme’s asymmetry. J Bioenerg Biomembr 27, 169–179 (1995). https://doi.org/10.1007/BF02110032
- Grandchamp, B., De Verneuil, H., Beaumont, C., Chretien, S., Walter, O. and Nordmann, Y. (1987), Tissue‐specific expression of porphobilinogen deaminase. European Journal of Biochemistry, 162: 105-110. doi:1111/j.1432-1033.1987.tb10548.x
- Battersby, A., Fookes, C., Matcham, G. et al. Biosynthesis of the pigments of life: formation of the macrocycle. Nature 285, 17–21 (1980). https://doi.org/10.1038/285017a0
- Paul R. Ortiz de Montellano (2008). “Hemes in Biology”. Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:1002/9780470048672.wecb221
- Straka, J.G., Kushner, J.P Purification and characterization of bovine hepatic uroporphyrinogen decarboxylase. Biochemistry 1983, 22, 20, 4664-4672. https://doi.org/10.1021/bi00289a009
- T. Yoshinaga, S. Sano Coproporphyrinogen oxidase: I. Purification, properties, and activation by phospholipids. Biol. Chem., 255 (10) (1980), pp. 4722-4726.
- Yoshinaga, S. Sano Coproporphyrinogen oxidase: II. Reaction mechanism and role of tyrosine residues on the activity. J. Biol. Chem., 255 (10) (1980), pp. 4727-4731.
- Dailey HA. 1990. Conversion of coproporphyrinogen to protoheme in higher eukaryotes and bacteria: Terminal three enzymes. In Biosynthesis of heme and chlorophylls (ed. Dailey HA), pp. 123–161. McGraw-Hill, New York.
- Dailey, T. A. & Dailey, H. A. Human protoporphyrinogen oxidase: Expression, purification, and characterization of the cloned enzyme. Protein Sci. 5, 98–105 (1996). https://doi.org/10.1002/pro.5560050112
Functionalization Chemistry of Protoporphyrin IX
Protoporphyrin IX is a versatile intermediate in the laboratory synthesis of many unique derivatives of protoporphyrin. The functionalization of protoporphyrin has allowed access to hundreds of new molecules with potential applications in medicine, materials science, and catalysis.12,13
Incorporation of Various Metals
- Mn(III) Protoporphyrin IX Chloride (manganese protoporphyrin)
- Tin protoporphyrins
- Chromium protoporphyrins
- Zn(II) Protoporphyrin IX (zinc protoporphyrin)
- (Co(III) Protoporphyrin IX (cobalt protoporphyrin) has been shown to inhibit heme oxygenase in-vitro but promotes heme oxygenase in in-vivo experiments16
- Sn(IV) Mesoporphyrin IX Dichloride (tin mesoporphyrin) and Cr(III) Mesoporphyrin IX Chloride (chromium mesoporphyrin) have both been shown to be inhibitors of heme oxygenase without photosensitization15.
- Zn(II) Deuteroporphyrin IX bis glycol (zinc deuteroporphyrin) has been foound to inhibit heme oxygenase in-vivo while having a short duration of action18
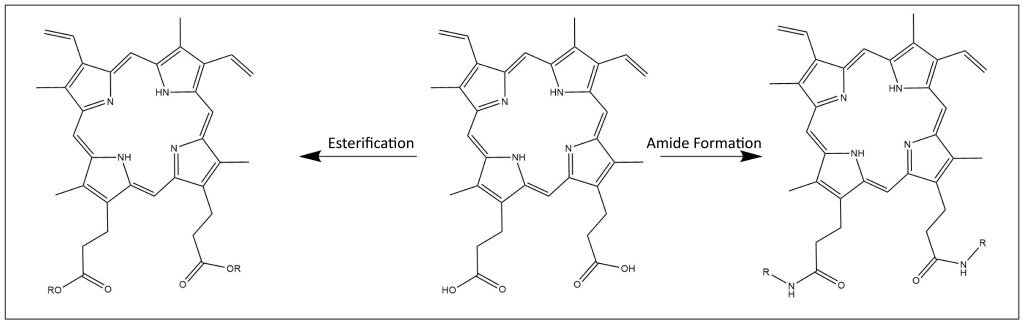
Modification of Carboxylic Acids
Protoporphyrin IX dimethyl ester is useful for further derivatization since it is much more soluble in organic solvents than protoporphyrin IX itself. In addition to ester forms, peptide coupling conditions can be used to produce bis-amides from PPIX for use in the field of photodynamic therapy research14,15.
Need protoporphyrin IX dimethyl ester?
We offer it in research and bulk quantities, ready to be shipped worldwide!
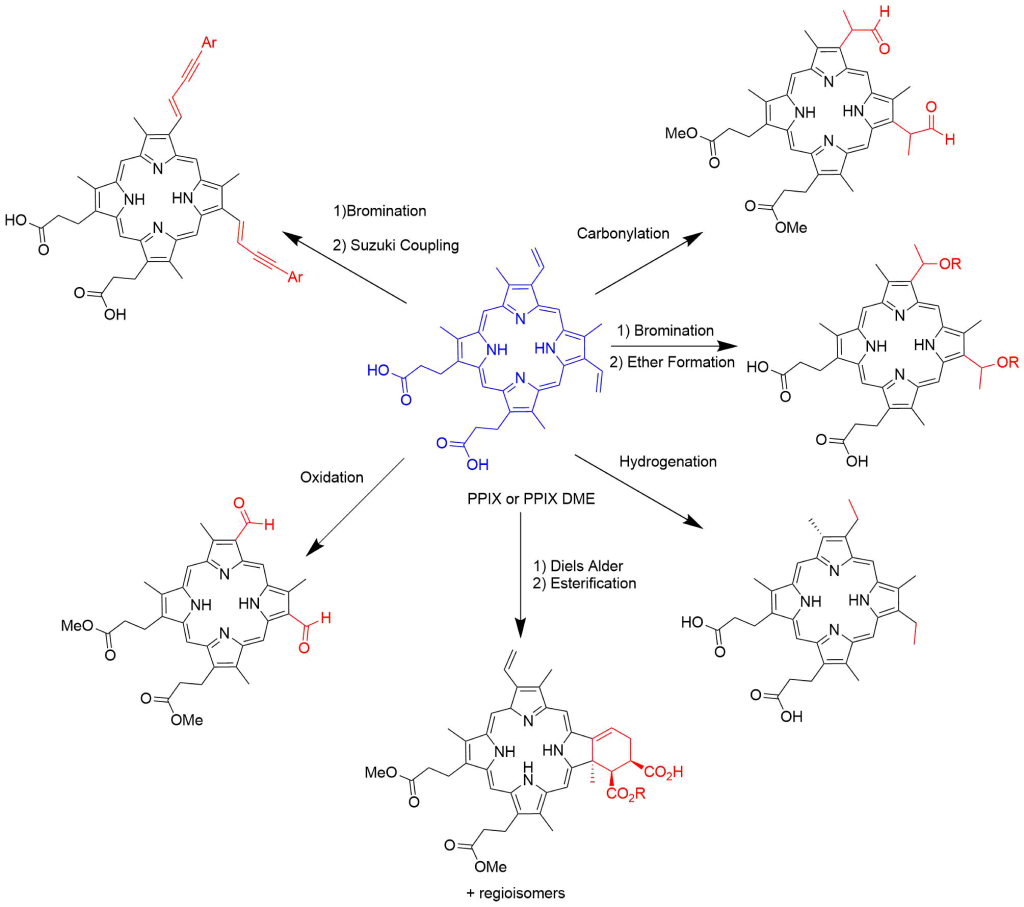
Modification of Vinyl Groups
Functionalization of the bis-vinyl groups can be accomplished by several different synthetic transformations. Bromination of the vinyl groups followed by the addition of alcohols gives access to mesoporphyrin derivatives that contain ether groups. Reduction of the vinyl groups affords mesoporphyrin IX as the product.
A multistep procedure has been reported for the conversion of both vinyl groups into the bis-aldehyde species17.
- Sitte, Elisabeth., Senge, Mathias O., The Red Color of Life Transformed – Synthetic Advances and Emerging Application of Protoporphyrin IX in Chemical Biology. Eur. J. Org. Chem. 2020, 3171-3191.
- Pavlov, V. Yu., Modern Aspects of the Chemistry of Protoporphyrin IX. Russian Journal of Organic Chemistry, 2007, 43, 1-34.
- Maximova, Ksenia., Pisarek, Sabina, Gryko, Dorota., A Practical Protocol for the Conjugation of various Amino Acids to Protoporphyrin IX. Synthesis 2013; 45, 1099-1105.
- Uchoa, Adjaci, F., Oliveira, Carla, S., Baptista, Mauricio, S. Relationship between structure and photoactivity of porphyrins derived from protoporphyrin IX. Journal of Porphyrins and Phthalocyanines, 2010, 14, 832-845.
- Peixoto, Andreia., Pareira, Mariette M., Neves, Graca M., Silva, Artur, M.S., Cavaleiro, Jose, A.S., Hydroformylation: a versatile tool for the synthesis of new B-formyl-metalloporphyrins. Tetrahedron Letters, 2003, 44, 5593-5595.
- Miyata, Kota., Yasuda, S., Masuya, Takuto., Ito, Satoshi., Kinoshita, Yusuke., Tamiaki, Hitoshi., Oba, Toru., Facile iodination of the vinyl groups in protoporphyrin IX dimethyl ester and subsequent transformation of the iodinated moieties. Tetrahedron, 2018, 74, 3707-3711.
- O’Brien, Jessica, M., Sitte, Elisabeth., Flanagan, Keith, J., Kuhner, Hannes., Hallen, Lukas j., Gibbons, Daire., Senge, Mathias O., Functionalization of Deutero- and Protoporphyrin IX Dimethyl Esters via Palladium-Catalyzed Coupling Reactions. J. Org. Chem. 2019, 84, 6158-6173.
- 19. de Oliveira, Kleber T., Silva, Artur M.S., Tome, Augusto C., Neves, Maria G.P.M.S., Neri, Claudio, R., Garcia, Vinicius S., Serra, Osvaldo, A., Iamamoto, Yassuko., Cavaleiro, Jose A.S., Synthesis of new amphiphilic chlorin derivatives from protoporphyrin-IX dimethyl ester. Tetrahedron, 2008, 64, 8709-8715.
Applications of Protoporphyrin IX
Protoporphyrin IX and its metalated derivatives have a myriad of applications due to unique biological activity20,21,22,23, acid-base and pi-pi stacking and optical properties.Heme oxygenase research & heme oxygenase inhibitors
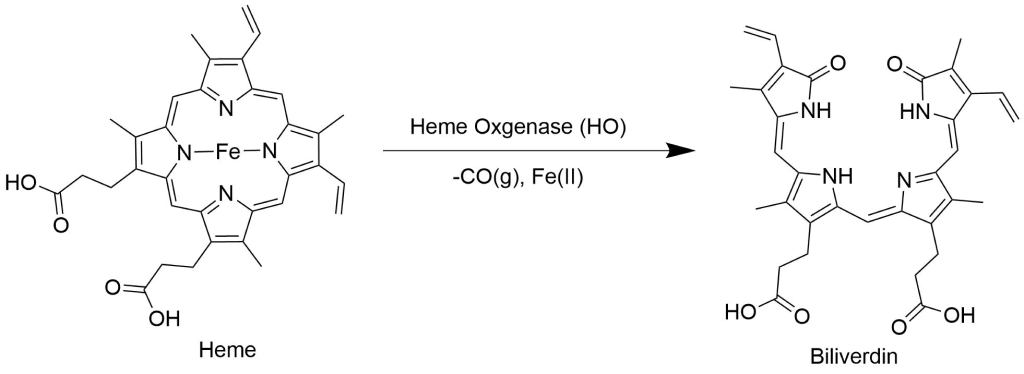
G-quadruplex-Protoporphyrin IX-based sensors:
Ferrochelatase Inhibitors:
Antimicrobial activity:
Ga(III) protoporphyrin IX, In(III) protoporphyrin IX, Co(III) protoporphyrin IX, Mn(III) protoporphyrin IX, and Cu(II) protoporphyrin IX exhibit antimicrobial activity against several pathogenic microorganisms such as Staphylococcus aureus, Mycobacterium abscessus, and Haemophilus influenzae. This activity is attributed to reduced uptake of heme or adverse properties of the non-native metalloporphyrins38,39,40.
Reconstitution of Metalloproteins:
- Vreman,J., Ekstrand, B.C., Stevenson, D.K. Selection of metalloporphyrin heme oxygenase inhibitors based on potency and photoreactivity. Pediatr Res. 1993, 33, 195-200. DOI: 10.1203/00006450-199302000-00021
- Schulz, S., Wong, R.J., Vreman, H.J., Stevenson, D.K. Metalloporphyrins – an update. Front. Pharmacol., 2012. https://doi.org/10.3389/fphar.2012.00068
- Drummond, G.S., Kappas, A. Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc. Natl. Acad. Sci. USA., 1981, 78, 6466-6470. https://doi.org/10.1073/pnas.78.10.6466
- Morioka, I., Wong, R., Abate, A. et al. Systemic Effects of Orally-Administered Zinc and Tin (IV) Metalloporphyrins on Heme Oxygenase Expression in Mice. Pediatr Res 59, 667–672 (2006). https://doi.org/10.1203/01.pdr.0000215088.71481.a6
- Kikuchi G, Yoshida T, Noguchi M (2005). “Heme oxygenase and heme degradation”. Biochem. Biophys. Res. Commun. 338 (1): 558–567. doi:1016/j.bbrc.2005.08.020
- Ahmad Z, Salim M, Maines MD (Mar 2002). “Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress”. The Journal of Biological Chemistry. 277 (11): 9226–32. doi:1074/jbc.M10823920
- Jansen, P.L.M., Mulder, G.J., Burchell, B. and Bock, K.W. (1992), New developments in glucuronidation research: Report of a workshop on “Glucuronidation, its role in health and disease”. Hepatology, 15: 532-544. doi:1002/hep.1840150328
- Ferreira GC, Franco R, Lloyd SG, Moura I, Moura JJ, Huynh BH. Structure and function of ferrochelatase. J Bioenerg Biomembr. 1995 Apr;27(2):221-9. doi: 10.1007/BF02110037
- Cole, S.P.C., Marks, G.S. Ferrochelatase and N-alkylated porphyrins. Mol Cell Biochem 64, 127–137 (1984). https://doi.org/10.1007/BF00224769
- Zhen Shi, Gloria C. Ferreira; Modulation of inhibition of ferrochelatase by N-methylprotoporphyrin. Biochem J 1 October 2006; 399 (1): 21–28. doi: https://doi.org/10.1042/BJ20060753
- F De Matteis, A H Gibbs, A G Smith; Inhibition of protohaem ferro-lyase by N-substituted porphyrins. Structural requirements for the inhibitory effect. Biochem J 1 September 1980; 189 (3): 645–648. doi: https://doi.org/10.1042/bj1890645
- Ida, J.; Chan, S.K.; Glökler, J.; Lim, Y.Y.; Choong, Y.S.; Lim, T.S. G-Quadruplexes as An Alternative Recognition Element in Disease-Related Target Sensing. Molecules 2019, 24, 1079. https://doi.org/10.3390/molecules24061079
- Z.-Z. Yang, Z.-B. Wen, X. Peng, Y.-Q. Chai, W.-B. Liang, R. Yuan, A novel fluorescent assay for the ultrasensitive detection of miRNA-21 with the use of G-quadruplex structures as an immobilization material for a signal indicator. Chem. Commun. 2019, 55, 6453-6456.
- Z. Zhang, E. Sharon, R. Freeman, X. Liu, I. Willner, Fluorescence Detection of DNA, Adenosine-5’-Triphosphate (ATP), and Telomerase Activity by Zinc(II)-Protoporphyrin IX/G-Quadruplex Labels. Anal. Chem. 2012, 84, 4789–4797;
- Yett, A., Lin, L.Y., Beseiso, D., Miao, J., Yatsunyk, L.A. N-methyl mesoporphyrin IX as a highly selective light-up probe for G-quadruplex DNA. Journal of Porphyrins and Phthalocyanines Vol. 23, No. 11n12, pp. 1195-1215 (2019) https://doi.org/10.1142/S1088424619300179
- Stone, K.L.; Hua, J.; Choudhry, H. Manganese-Substituted Myoglobin: Characterization and Reactivity of an Oxidizing Intermediate towards a Weak C-H Bond. Inorganics 2015, 3, 219-229. https://doi.org/10.3390/inorganics3020219
- Key, H., Dydio, P., Clark, D. et al. Abiological catalysis by artificial haem proteins containing noble metals in place of iron. Nature 534, 534–537 (2016). https://doi.org/10.1038/nature17968
- Teruyuki Komatsu, Rong-Min Wang, Patricia A. Zunszain, Stephen Curry, and Eishun Tsuchida Journal of the American Chemical Society 2006 128 (50), 16297-16301 DOI: 10.1021/ja0656806
- Stojiljkovic I, Kumar V, Srinivasan N. Non-iron metalloporphyrins: potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol Microbiol. 1999 Jan;31(2):429-42. doi: 10.1046/j.1365-2958.1999.01175.x. PMID: 10027961.
- Morales-de-Echegaray, A.V., Maltais, T.R., Lin, L., Younis, W., Kadasala, N.R., Seleem, M.N., Wei, A. ACS Infect. Dis. 2018, 4, 11, 1564-1573. https://doi.org/10.1021/acsinfecdis.8b00125
- Visaggio, D., Frangipani, E., Hijazi, S., Pirolo, M., Leoni, L., Rampioni, G., Imperi, F., Bernstein, L., Sorrentino, R., Ungaro, F., Visca, P. ACS Infect. Dis. 2022, 8, 1, 78-85. 10.1021/acsinfecdis.1c00409

