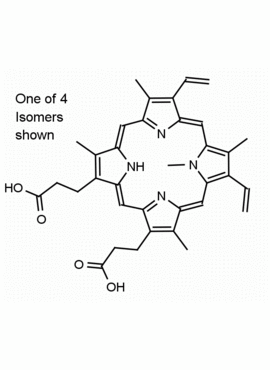
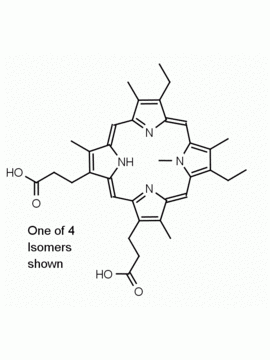
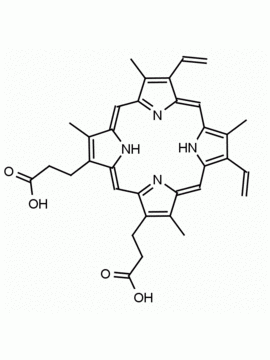
N-Methyl Protoporphyrin IX CAS: 79236-56-9 MDL: MFCD08669600
Background: N-Methyl Protoporphyrin IX is a transition state analogue and a potent inhibitor of ferrochelatase, the enzyme that inserts iron into protoporphyrin IX and is commonly used to induce haem deficiency in cell cultures.1
Molecular weight: 576.69 g/mol
Molecular Formula: C35H36N4O4
CAS Number: 79236-56-9
Storage: Store at 0 – 4 °C, protect from light.
Synonyms: N-Methyl Protoporphyrin IX, 3,3′-(7,12-diethyl-3,8,13,17,22-pentamethyl-22,24-dihydroporphyrin-2,18-diyl)dipropanoic acid
Fields of Interest: Heme Biosynthesis, Ferrochelatase Inhibitors, Photodynamic Therapy, Nanocomposites, Quantum Oxygen Yield, Catalysis, Photocatalysis
- Modulation of inhibition of ferrochelatase by N-methylprotoporphyrin, Shi, Z.; Ferreira, Gloria, C., Biochem J., 2006, 399, 21-28.
- Regulation of soluble guanylate cyclase activity by porphyrins and metalloporphyrins, Ignarro, Louis J.; Ballot, Bryan; Wood, Keith S., Journal of Biological Chemistry (1984), 259(10), 6201-7.
- Bovine ferrochelatase. Kinetic analysis of inhibition by N-methylprotoporphyrin, manganese, and heme, Dailey, Harry A.; Fleming, Jennie E., Journal of Biological Chemistry (1983), 258(19), 11453-9.
- Three-Dimensional Structure-Activity Analysis of a Series of Porphyrin Derivatives with Anti-HIV-1 Activity Targeted to the V3 Loop of the gp120 Envelope Glycoprotein of the Human Immunodeficiency Virus Type 1, Asim Kumar; Jiang, Shibo; Strick, Nathan; Lin, Kang; Haberfield, Paul; Neurath, A. Robert, Journal of Medicinal Chemistry (1994), 37(8), 1099-108DOI:10.1021/jm00034a007
- Acquisition of iron from transferrin regulates reticulocyte heme synthesis, Ponka, Premysl; Schulman, Herbert M., Journal of Biological Chemistry (1985), 260(27), 14717-21.
- Bovine ferrochelatase. Kinetic analysis of inhibition by N-methylprotoporphyrin, manganese, and heme , Dailey H A; Fleming J E, The Journal of biological chemistry (1983), 258(19), 11453-9,
- Heme deficiency is associated with senescence and causes suppression of N-methyl-D-aspartate receptor subunits expression in primary cortical neurons, Chernova, Tatyana; Nicotera, Pierluigi; Smith, Andrew G., Molecular Pharmacology (2006), 69(3), 697-705. DOI:10.1124/mol.105.016675
- Recombinant cytochromes c biogenesis systems I and II and analysis of haem delivery pathways in Escherichia coli, Feissner Robert E; Richard-Fogal Cynthia L; Frawley Elaine R; Loughman Jennifer A; Earley Keith W; Kranz Robert G, Molecular microbiology (2006), 60(3), 563-77
- Characterization of porphyrin interactions with peripheral type benzodiazepine receptors, Verma, Ajay; Snyder, Solomon H., Molecular Pharmacology (1988), 34(6), 800-5.
- Prediction of Anti-HIV-1 Activity of a Series of Tetrapyrrole Molecules, Vanyur, Rozalia; Heberger, Karoly; Jakus, Judit, Journal of Chemical Information and Computer Sciences (2003), 43(6), 1829-1836. DOI:10.1021/ci0304627
- Peripheral benzodiazepine receptor ligands in rat liver mitochondria: effect on cholesterol translocation, Tsankova, Virginia; Magistrelli, Armando; Cantoni, Lavinia; Tacconi, Maria Teresa, European Journal of Pharmacology (1995), 294(2/3), 601-7. DOI:10.1016/0014-2999(95)00603-6