Chemical and biological sensors are becoming more and more ubiquitous. They are utilized not only in science and academics, but in medicine, manufacturing and numerous other industries. Most people interact with some kind of chemical or biological sensor, knowingly or not, as they go about their lives. Sensors help us to monitor, analyze and understand scientific processes, help to detect and abate dangerous toxins and chemicals, assist in keeping food and water safe, and play an important role in medicine and public health. There are a myriad of sensors that utilize a vast variety of chemical receptors. At Frontier Specialty Chemicals, we specialize in the chemistry of boronic acids and porphyrins. These chemicals are employed as key components in many chemical and biological sensors used to analyze an ever-expanding array of analytes.
Boronic Acid-based Sensors
The reversible formation of covalent bonds between boronic acids and diols has led to boronic acids being increasingly implemented in sensors for saccharides.
Many fluorescent sensors for saccharides have been developed based on fluorophore appended boronic acids. The intensity of fluorescence of the fluorophore can be significantly increased or decreased or even quenched when a saccharide binds to the boronic acid receptor. More complex boronic acid derivatives of fluorescent compounds have been synthesized and employed in sensors that allow for selective detection of saccharides. In one such example, Arimori et al. used a boronic acid functionalized porphyrin in a competitive assay system for the selective fluorescent detection of D-fructose.6
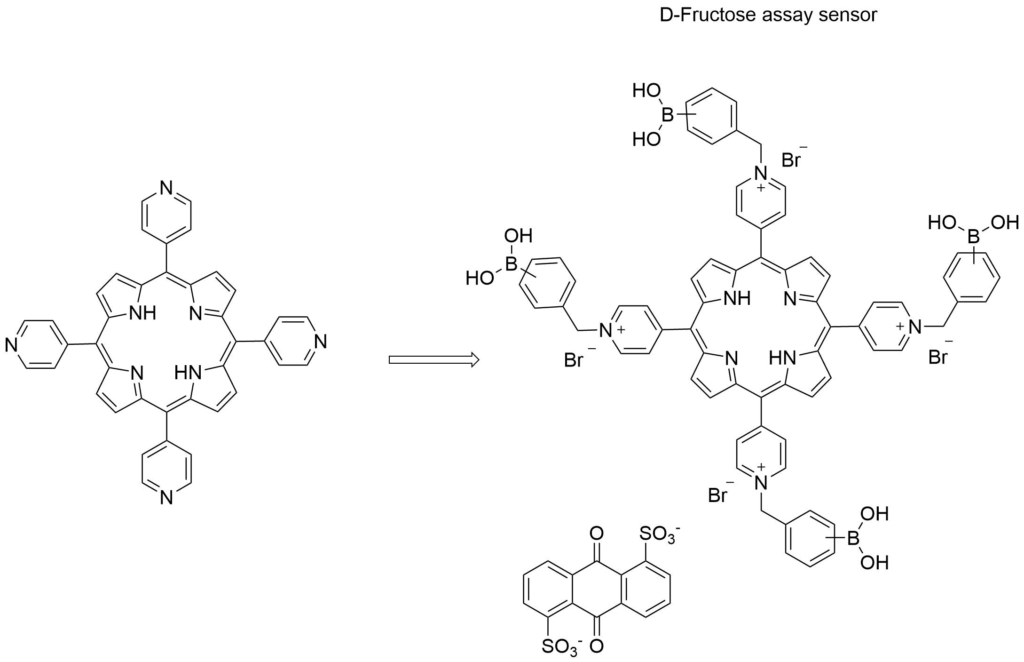
| Catalog Number: T618 |
Boronic acids are Lewis acids and readily interact with Lewis bases. As such, they have been implemented in sensors to detect a variety of anions, including fluoride and cyanide.
Yoon, et. al prepared a boronic acid derivative of a fluorescein motif that showed a large fluorescence increase in the presence of fluoride anions.7
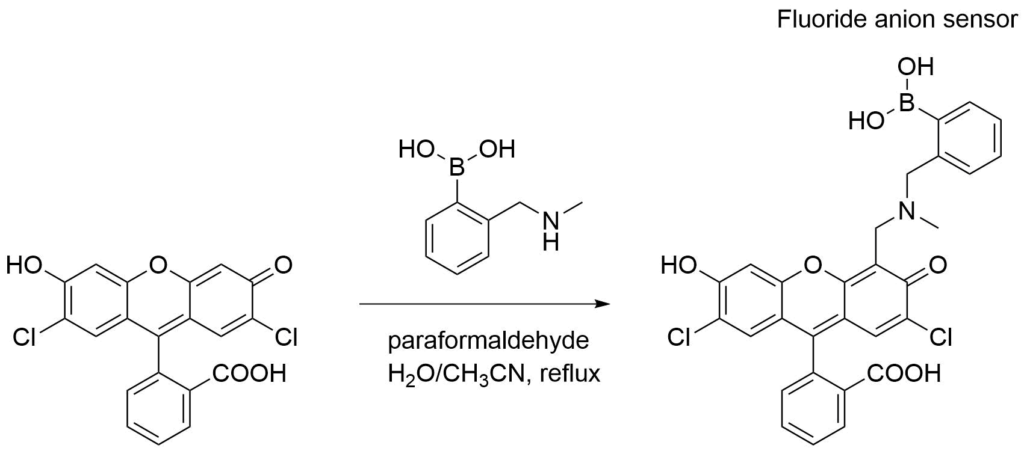
Porphyrin-based Sensors
Porphyrins have many unique properties related to colorimetry, spectrophotometry, and photo-electrochemistry. Because of this, porphyrins, especially metalloporphyrins, are utilized in a wide variety of sensors used to detect everything from volatile organic compounds (VOCs), reactive oxygen species (ROS) and metal ions to pathogens, toxic industrial chemicals, and even explosives.8,9,10
As a world leader in porphyrin synthesis, Frontier Scientific offers catalog and custom synthesis of an expansive array of porphyrins utilized in these types of applications. A myriad of other tetrabenzo and tetranaphthoporphine analogues are available in our online catalog and by inquiry.
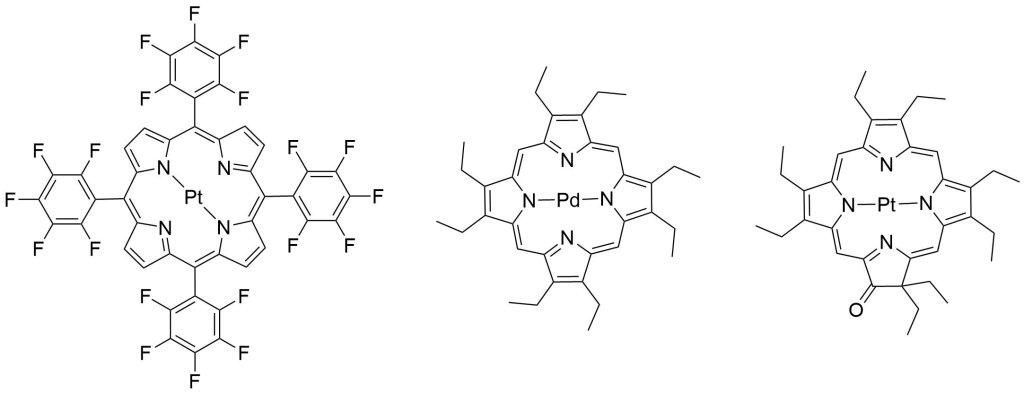
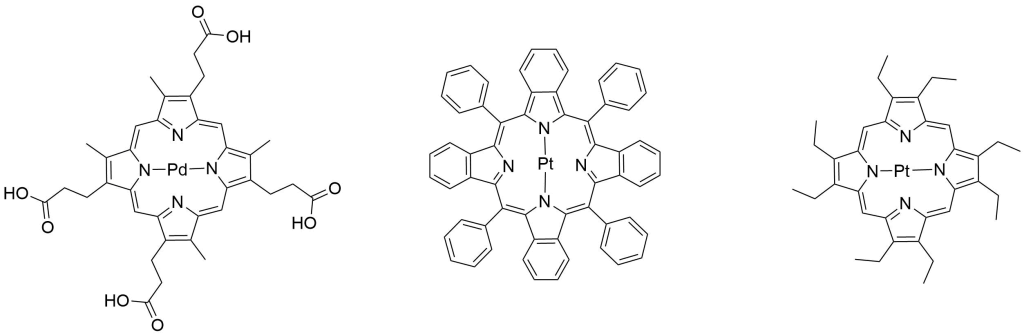
References
- J. Yoon, A. W. Czarnik, J. Am. Chem. Soc. 1992, 114, 5874-5875.
- J. Yoon, A. W. Czarnik, Bioorg. Med. Chem. 1993, 1, 267-271.
- Y. Nagai, K. Kobayashi, H.Toi, Y. Aoyama, Bull. Chem. Soc. Jpn. 1993, 66, 2965-2971.
- H. Suenaga, M. Mikami, K. R. A. S. Sandanayake, S. Shinkai, Tetrahedron Lett. 1995, 36, 4825-4828.
- H. Suenaga, H. Yamamoto, S. Shinkai, Pure Appl. Chem. 1996, 68, 2179-2186.
- J. Chem. So. Chem. Commun., 1995, 961-962
- J. Org. Chem., 2006, 71, 8626-8628
- Chem. Rev. 2017, 117, 4, 2517-2583
- Platinum Metals Rev., 1997, 41, 115-127
- New J. Chem., 2018,42, 7529-7550