PDT is a binary cancer therapy, which relies on selective uptake of a photosensitizer into the cancer cells which upon irradiation with light of an appropriate wavelength produce highly toxic singlet oxygen and other cytotoxic species.1,2 Singlet oxygen can readily react with electron rich biomolecules such as unsaturated lipids, amino acids and DNA. Ultimately, these destructive reactions lead to cell death through apoptosis or necrosis.3
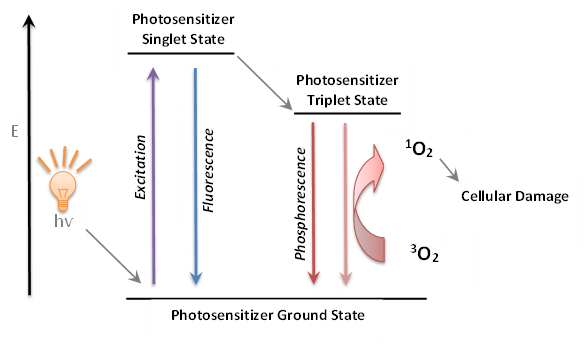
The following attributes of the porphyrin derivatives make them powerful and versatile chromophores for PDT studies:
1) The presence of main absorption bands in the therapeutic window, the Q bands, around 600-700 nm. It is advantageous to use red-shifted chromophores in order to get better penetration of exciting laser source;
2) Intense extinction coefficients. Singlet oxygen production depends on the extinction coefficient of the chromophore; and
3) Ease of modification of substituent groups. Porphyrins have several distinct functionalization sites, i.e., the meso position, β-position. The solubility of porphyrin derivatives in nonpolar or polar solvents can be modified by varying the porphyrin substituents.
These properties of porphyrin-based photosensitizers allow for deeper photodamage of tissue and a better therapeutic effect compared with non-porphyrinoids.
To overcome the major drawbacks in porphyrin based photosensitizers, low excitation coefficient in the therapeutic window and persistence in skin, so called second generation chlorin based photosensitizers, such as Foscan (Temoporfin), Talaporfin (LS11, N-aspartylchlorin e6) and HPPH (2-(1-hexyloxyethyl)-2-devinyl-pyropheophorbide a) have been developed.4 Chlorins are particularly promising photosensitizers for PDT because of their distinctive ability to accumulate in tumorous tissues,5 intense absorptions at 664 nm, high stability, low dark toxicity and ability to generate singlet oxygen in the light.6,7,8 Amphiphilicity, another favorable trait, has been shown to improve the effectiveness of photosentizers. Ce6 a derivative of chlorophyll a, possess three carboxylic side chains, making it an ideal substrate for synthesis of novel amphiphilic photosensitizers.
Porphyrin based PDT Photosensitizers
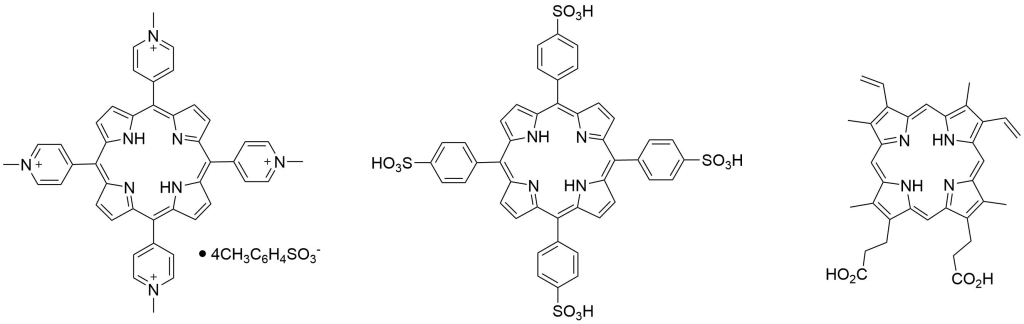
| Catalog Number: TMP-1363 TMPyP4 Tosylate | Catalog Number: T1239 TPPS4-2HCl | Catalog Number: P562-9 Protoporphyrin IX |
Chlorin based PDT Photosensitizers
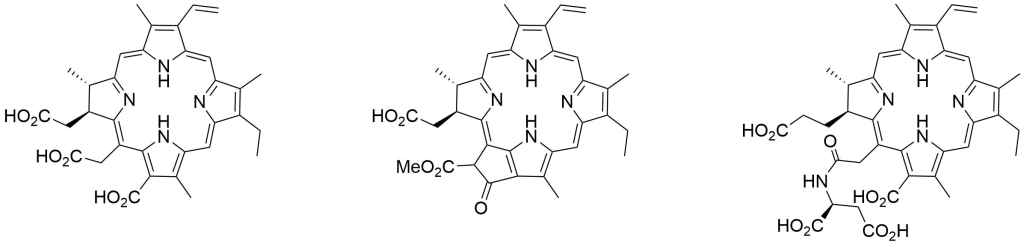
| Catalog Number: Ce6 Chlorin e6 | Catalog Number: Pha-592 Pheophorbide a | Catalog Number: A41107 Talaporfin, NPe6 |
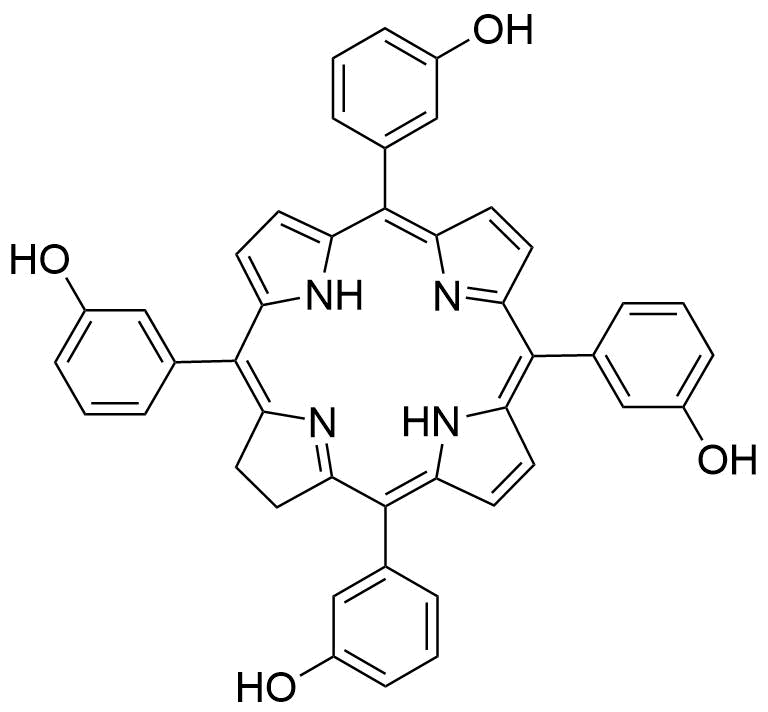
| Catalog Number: T40284 meso-tetra(m-hydroxyphenyl)chlorin (Foscan) |
Talaporfin Derivatives
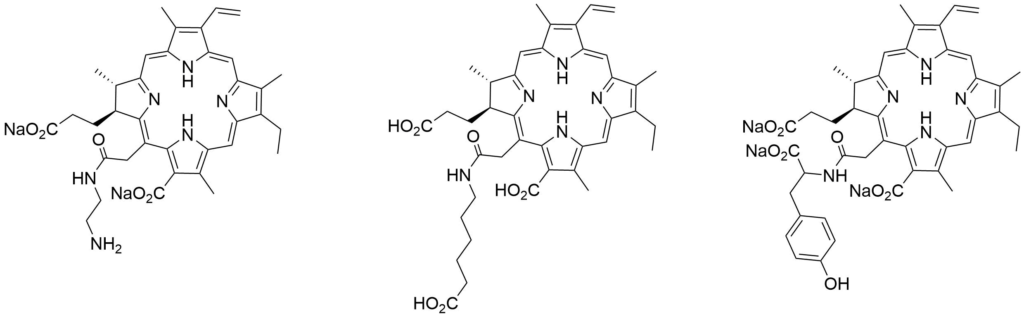
| Catalog Number: C40890 Ce6 mono ethylenediamine amide disodium salt | Catalog Number: C40286 Ce6 mono aminohexanoic amide trisodium salt | Catalog Number: M40403 Ce6 mono tyrosine amide trisodium salt |
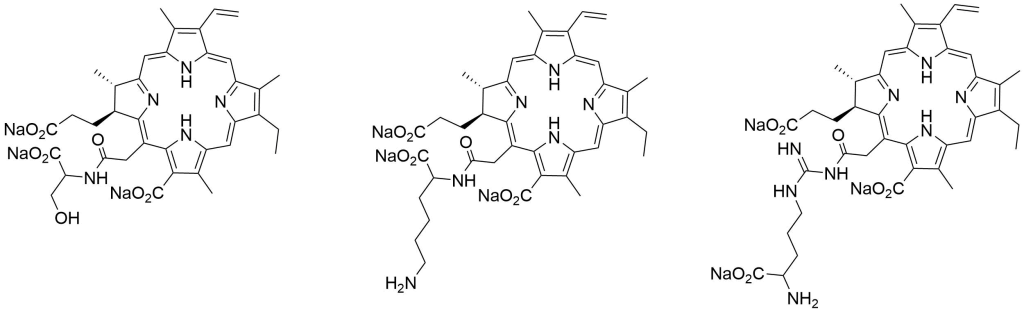
| Catalog Number: C40286 Ce6 mono serine amide trisodium salt | Catalog Number: C10409 Ce6 lysine amide trisodium salt | Catalog Number: C40402 Ce6 mono arginine amide trisodium salt |
References
1.) Dolmans, D. E. J. G. J.; Fukumura, D.; Jain, R. K. Nat. Rev. Cancer 2003, 3, 380.
3.) Vicente, M. G. H. Curr. Med. Chem. AntiCancer Agents 2001, 1.
6.) Kostenicha, G. A.; Zhuravkina, I. N.; Zhavrid, E. A. J. Photochem. Photobiol. B. 1994, 22, 211.
7.) Kessel, D. Photochem. Photobiol. 2008, 49, 447.
8.) Palma, M.; Cárdenas-Jirón, G.; Menéndez, R. M. I., M. J. Phys. Chem. A 2008, 112,13574.