
Numerous applications of porphyrin derivatives in catalysis are known. Metalloporphyrins act as cytochrome P-450 mimics in various chemistries and can be tuned for high selectivity and low catalyst loading. Frontier Specialty Chemicals provides numerous metalloporphyrins for catalytic applications. Mimics of cytochrome P-450 based upon metalloporphyrins are capable of selective C-H oxidative functionalization, epoxidation, sulfide to sulfoxide oxidation without the use of explosive organoperoxyacids, strong mineral acids or toxic metal oxo complexes. Free base porphyrins are also useful for photoredox catalysis and photosensitizers.
Selective C-H azidation:
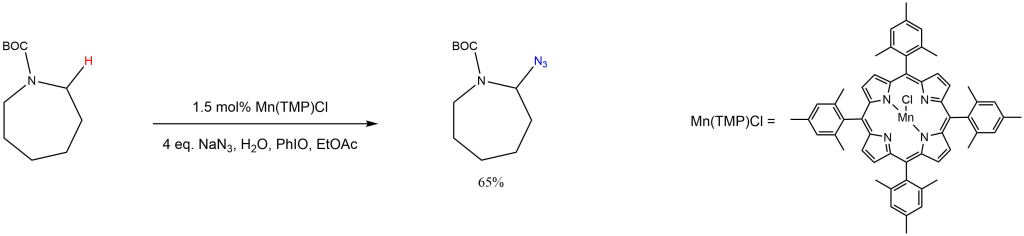
Reported by the Groves group: J. Am. Chem. Soc. 2015, 137, 5300-5303
Selective C-H fluorination:
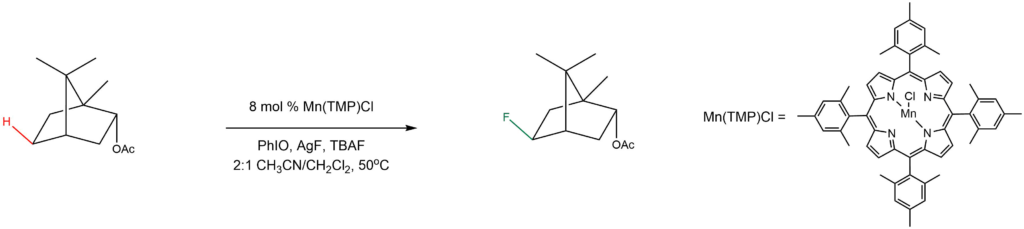
Reported by the Groves group: Nature Protocols 2013, 8, 2348-2354
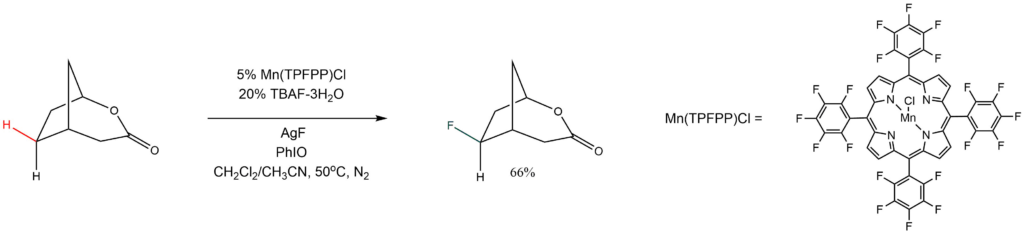
Reported by the Groves group: Angew. Chem. Int. Ed. 2018, 57, 1251-1255
Epoxidation:
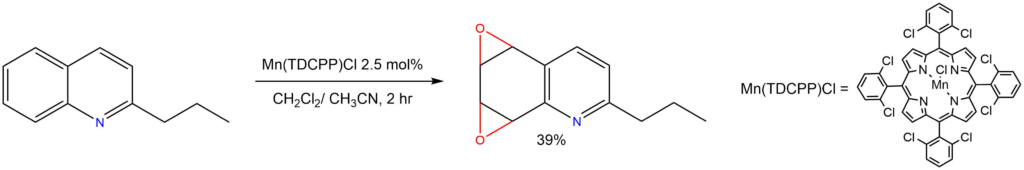
Reported in : Org. Biomol. Chem. 2008, 6, 4494-4497
Porphyrins as photosensitizers for endoperoxide formation:
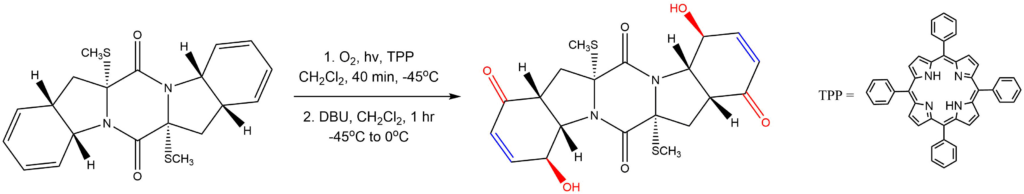
Reported by the Nicolaou group: J. Am. Chem. Soc. 2011, 133, 8150-8153
Intramolecular C-H amination of azides:
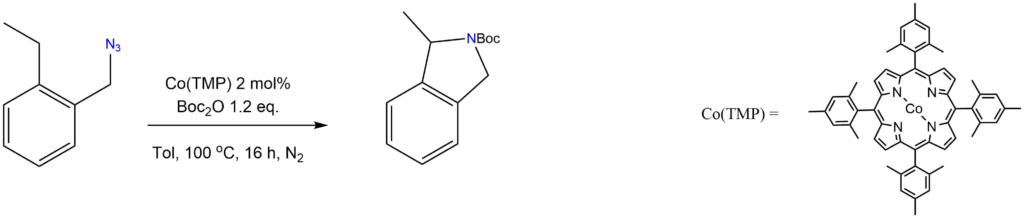
Reported by the de Bruin group: Chem. Eur. J. 2017, 23, 7945-7952
Tryptamine synthesis by C-H functionalization of indoles:
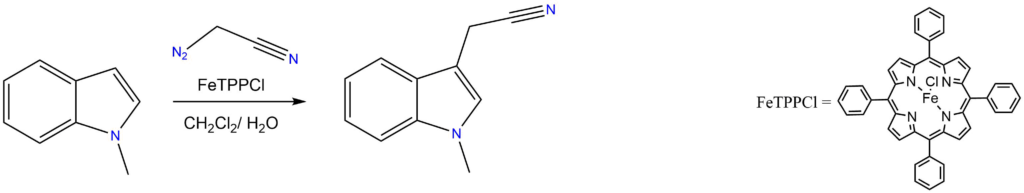
Reported by the Koenigs group: Angew. Chem. Int. Ed. 2019, 58, 3630-3634
Miyaura borylation by Pd(II) porphyrin catalysts:
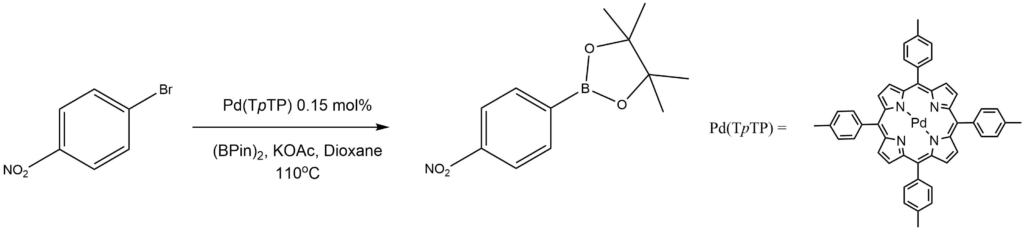
Reported by the Venkateswarlu group: Synlett 2018, 29, 1055-1060
Cis-selective hydrogenation of alkynes:
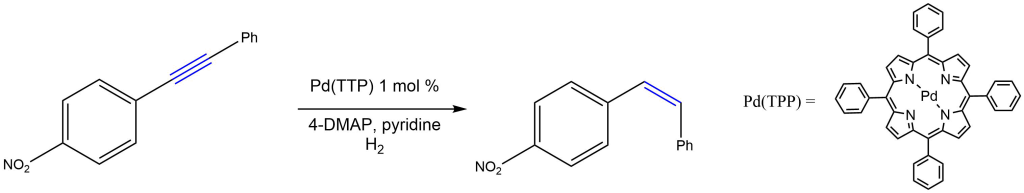
Reported by the Matsubara group: Synlett 2014, 25, 1287-1290
Oxidative coupling of phenols:
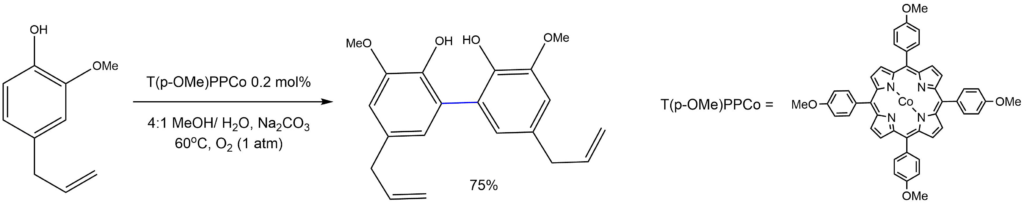
Reported by the Guo group: Eur. J. Org. Chem. 2013, 10, 1861-1866
Cyclopropanation with in-situ generated trifluoromethyl diazomethane:
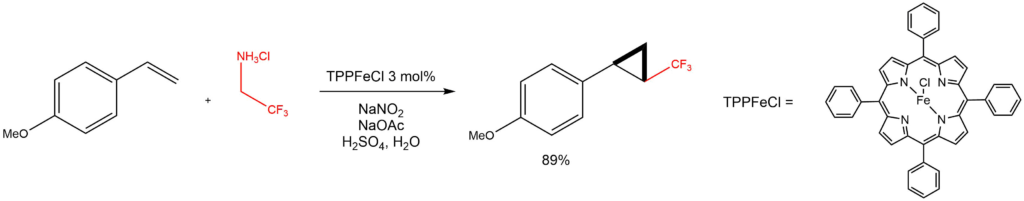
Reported by the Carreira group: Angew. Chem. Int. Ed. 2010, 49, 938-941
Cycloisomerization of allenones with chlorogoldtetraphenylporphine:
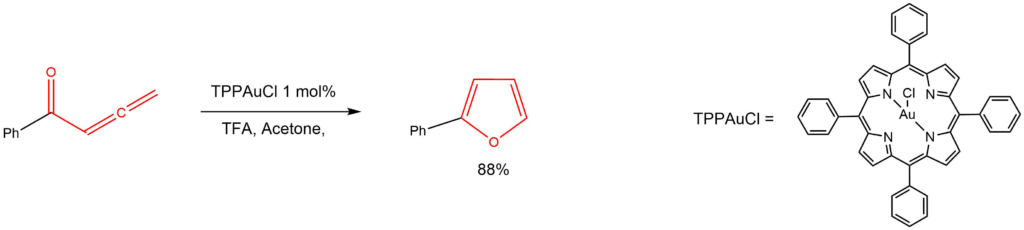
Reported by the Che group: Org. Lett. 2006, 8, 325-328
Carbonylation of epoxides using chlorochromiumtetraphenylporphine:
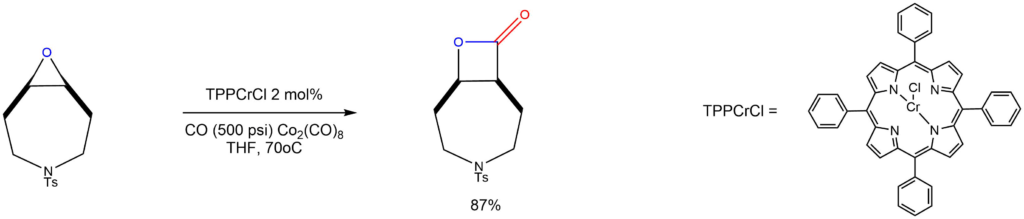
Reported by the Ibrahim group: Org. Lett. 2011, 13, 3142-3145