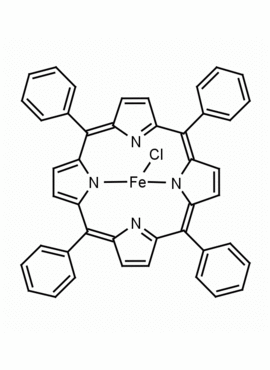
Fe(III) meso-Tetraphenylporphine Chloride (contains 1-3% chlorin) CAS: 16456-81-8 MDL: MFCD00012152
Molecular weight: 704.018 g/mol
Molecular Formula: C44H28ClFeN4
CAS Number: 16456-81-8
Storage: Store at room temperature, protected from light
Synonyms: 16456-81-8, Chlor[5,10,15,20-te
Field of Interest: Organic Synthesis, Olefination, Cyclopropanation, Aziridination, N-H Insertion, Oxidation,
Background: Fe(III) meso-Tetraphenylporphine Chloride is a specialty chemical manufactured by Frontier Specialty Chemicals which is useful for numerous catalytic organic transformations. Fe(III) meso-Tetraphenylporphine Chloride catalyzes a highly E-selective olefination of aldehydes with ethyl diazo acetate in the presence of PPh3.1 Selective Baeyer-Villiger oxidation of cycloketones to lactones with molecular oxygen was catalyzed by Fe(III) meso-Tetraphenylporphine Chloride with benzaldehyde as an oxygen acceptor.2 Fe(III) meso-Tetraphenylporphine Chloride catalyzes the cyclopropanation of styrenes via the in-situ generation of trfluoromethyldiazomethane.3 Fe(III) meso-Tetraphenylporphine Chloride catalyzes the N-H insertion of amines with ethyl diazo acetate.4
References:
1.) Chen, et al. Iron(III) and Ruthenium(II) Porphyrin Complex-Catalyzed Selective Olefination of Aldehydes with Ethyl Diazoacetate. J. Org. Chem. 2003, 68, 9, 3714–3717
2.) Zhou, et al. Baeyer-Villiger oxidation of ketones catalyzed by iron(III) meso-tetraphenylporphyrin chloride in the presence of molecular oxygen. Journal of Porphyrins and Phthalocyanines Vol. 12, No. 02, pp. 94-100 (2008)
4.) Baumann, et al. Iron Porphyrin Catalyzed N−H Insertion Reactions with Ethyl Diazoacetate. Organometallics 2007, 26, 16, 3995–4002