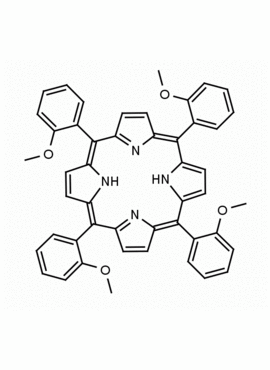
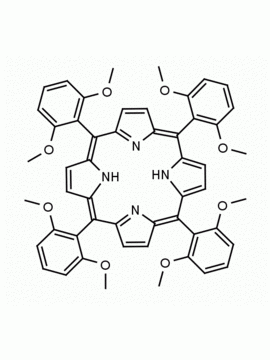
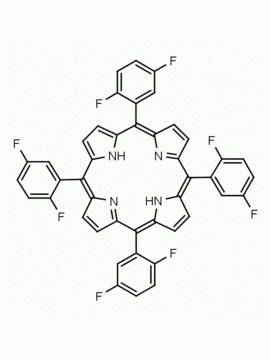
meso-Tetra(2-methoxyphenyl) porphine CAS: 29114-94-1 MDL: MFCD01074424
Molecular weight: 734.84 g/mol
Molecular Formula: C48H38N4O4
CAS Number: 29114-94-1
Storage: Store at room temperature, protect from light
Synonyms: 21H,22H-Porphine, 5
Field of Interest: Synthetic Porphyrins, Catalysis, Porphyrin Cage Compounds
Background: meso-Tetra(2-methoxyphenyl) porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. meso-Tetra(2-methoxyphenyl) porphine is sold as a mixture of atropisomers.
References:
1.) Chen, et al. Mechanistic Studies on the Epoxidation of Alkenes by Macrocyclic Manganese Porphyrin Catalysts. European Journal of Organic Chemistry, 2022, vol. 2022, # 35, art. no. E202200280.
2.) Shen, HM., Qi, B., Hu, MY. et al. Selective Solvent-Free and Additive-Free Oxidation of Primary Benzylic C–H Bonds with O2 Catalyzed by the Combination of Metalloporphyrin with N-Hydroxyphthalimide. Catal Lett 150, 3096–3111 (2020). https://doi.org/10.1007/s10562-020-03214-y
3.) Rapid and scalable synthesis of chiral porphyrin cage compounds. Tetrahedron, 2019, vol. 75, # 33, p. 4640 – 4647. https://doi.org/10.1016/j.tet.2019.07.009
4.) Momenteau, et al. Both-faces Hindered Porphyrins. Part 1. Synthesis and Characterization of Basket-handle Porphyrins and Their Iron Complexes. Journal of the Chemical Society. Perkin transactions I, 1983, # 1, p. 189 – 196. https://doi.org/10.1039/P19830000189
5.) Sugiyasu, et al. Conducting polymer networks cross-linked by “isolated” functional dyes: Design, synthesis, and electrochemical polymerization of doubly strapped light-harvesting porphyrin/oligothiophene monomers. Chemistry – A European Journal, 2009, vol. 15, # 26, p. 6350 – 6362. https://doi.org/10.1002/chem.200900576