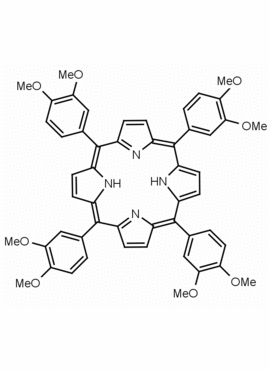
meso-Tetra(3,4-dimethoxyphenyl) porphine CAS: 88422-69-9 MDL: MFCD00830316
Molecular weight: 854.94 g/mol
Molecular Formula: C52H46N4O8
CAS Number: 88422-69-9
Storage: Store at room temperature, protect from light.
Synonyms: 1,2-Dimethoxy-4-[7,
Fields of Interest: Synthetic Porphyrins
Background: meso-Tetra(3,4-dimethoxyphenyl) porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Adineh, et al. Fabrication and analysis of dye-sensitized solar cells (DSSCs) using porphyrin dyes with catechol anchoring groups. RSC Advances, 2016, vol. 6, # 18, p. 14512 – 14521. https://doi.org/10.1039/C5RA23584G
2.) Katona, et al. Triplet state spectroscopic studies on some 5,10,15,20-tetrakis(methoxyphenyl)porphyrins. Journal of Molecular Structure, 1998, vol. 450, # 1-3, p. 41 – 45. https://doi.org/10.1016/S0022-2860(98)00411-6
3.) Sergeeva, et al. Synthesis of hydroporphyrins based on comparative studies of palladium-catalyzed and non-catalyzed approaches. Tetrahedron, 2007, vol. 63, # 50, p. 12454 – 12464. https://doi.org/10.1016/j.tet.2007.09.030
4.) Marydasan, et al. In Vitro and in Vivo Demonstration of Human-Ovarian-Cancer Necrosis through a Water-Soluble and Near-Infrared-Absorbing Chlorin. Journal of Medicinal Chemistry, 2018, vol. 61, # 11, p. 5009 – 5019. https://doi.org/10.1021/acs.jmedchem.8b00460
5.) Shehzad, et al. Organic-Inorganic Hybrids Composed of Keggin-Type [PMo10V2O40]5- Anions and Porphyrins: The Synthesis, Characterization, and Influence of Porphyrin Substituents on Optical Nonlinearities. Journal of Physical Chemistry C, 2020, vol. 124, # 17, p. 9442 – 9450. https://doi.org/10.1021/acs.jpcc.0c00861
6.) Chen, et al. Energy Band Alignment and Redox-Active Sites in Metalloporphyrin-Spaced Metal-Catechol Frameworks for Enhanced CO2 Photoreduction. Angewandte Chemie – International Edition, 2022, vol. 61, # 1, art. no. E202111622. https://onlinelibrary.wiley.com/doi/epdf/10.1002/anie.202111622