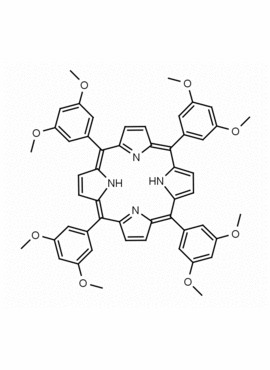
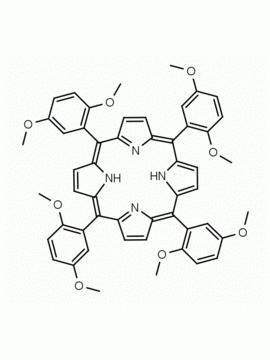
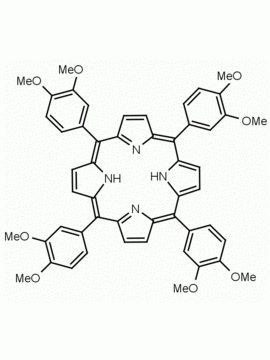
meso-Tetra (3,5-dimethoxyphenyl) porphine CAS: 74684-34-7 MDL: MFCD02093501
Molecular weight: 854.94 g/mol
Molecular Formula: C52H46N4O8
CAS Number: 74684-34-7
Storage: Store at room temperature, protect from light.
Synonyms: 21H,23H-Porphine, 5
Fields of Interest: Synthetic Porphyrins, Dendrimers
Background: meso-Tetra (3,5-dimethoxyphenyl) porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Okada, et al. Substituent-Control Exciton in J-Aggregates of Protonated Water-Insoluble Porphyrins. J. Am. Chem. Soc. 2003, 125, 9, 2792–2796. https://doi.org/10.1021/ja017768j
2.) Dichtel, et al. Functionally Layered Dendrimers: A New Building Block and Its Application to the Synthesis of Multichromophoric Light-Harvesting Systems. Org. Lett. 2005, 7, 20, 4451–4454. https://doi.org/10.1021/ol0516824
3.) Pollak, et al. A comparison of two convergent routes for the preparation of metalloporphyrin-core dendrimers: direct condensation vs. chemical modification. J. Mater. Chem., 1998,8, 519-527. https://doi.org/10.1039/A705410F
4.) Sharma, et al. Hypervalent Phosphorus(V) Porphyrins with meso-Methoxyphenyl Substituents: Significance of the Number and Position of Methoxy Groups in Promoting Intramolecular Charge Transfer. Inorg. Chem. 2022, 61, 42, 16573–16585. https://doi.org/10.1021/acs.inorgchem.2c01648
5.) Wakahara, et al. One-Dimensional Fullerene/Porphyrin Cocrystals: Near-Infrared Light Sensing through Component Interactions. ACS Appl. Mater. Interfaces 2020, 12, 2, 2878–2883. https://doi.org/10.1021/acsami.9b18784
6.) Dar, et al. Robust and electron deficient oxidovanadium(iv) porphyrin catalysts for selective epoxidation and oxidative bromination reactions in aqueous media. Green Chem., 2019,21, 1757-1768. https://doi.org/10.1039/C8GC03909G
7.) Zheng, et al. Design of two-photon absorbing fluorophores for FRET antenna-core oxygen probes. New J. Chem., 2018,42, 7914-7930. https://doi.org/10.1039/C7NJ05073A
8.) Cabrera-Gonzalez, et al. High-Boron-Content Porphyrin-Cored Aryl Ether Dendrimers: Controlled Synthesis, Characterization, and Photophysical Properties. Inorg. Chem. 2015, 54, 10, 5021–5031. https://doi.org/10.1021/acs.inorgchem.5b00618