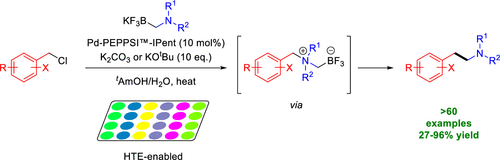
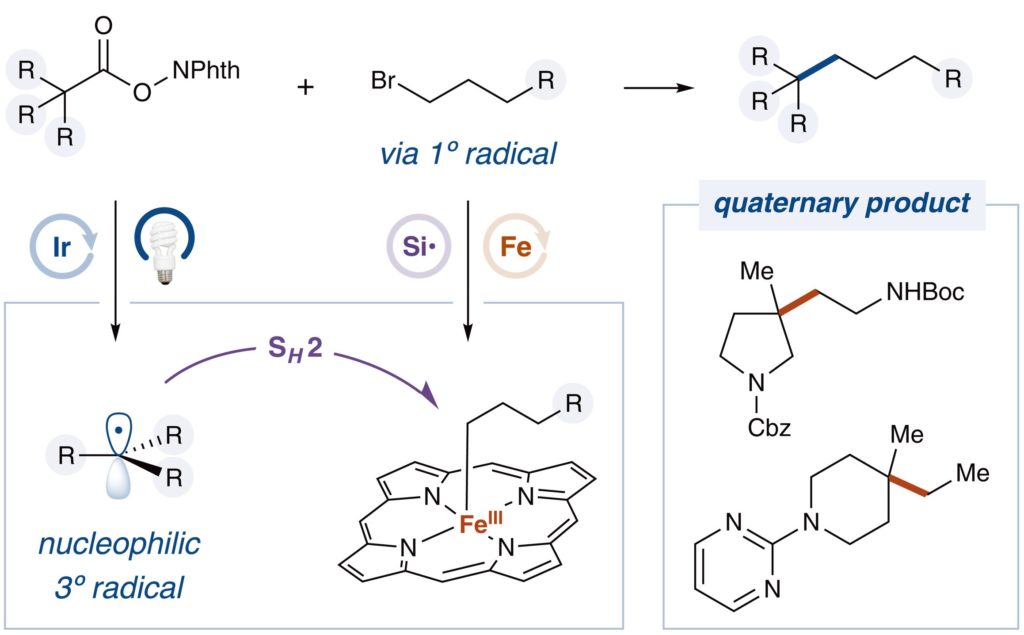
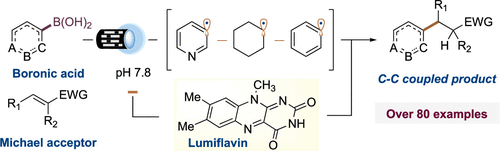
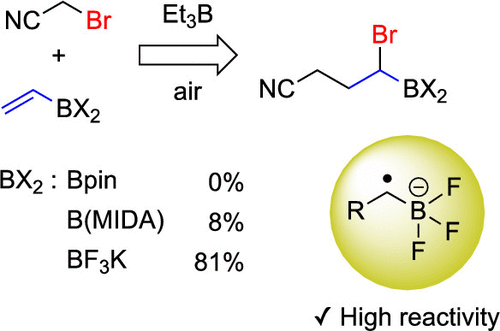
Synthesis of Arylethylamines via C(sp3)–C(sp3) Palladium-Catalyzed Cross-Coupling
Research published in The Journal of Organic Chemistry, from Lippa, R. et al., has shown the access to substituted arylethylamines that are key structural components in natural, pharmaceutical, and agrochemical compounds. Substituted phenylethylamine and structurally related analogues represent a key pharmacophore found in endogenous and synthetic neurotransmitters, pharmaceutical agents, and agrochemical products including products such […]
Synthesis of Arylethylamines via C(sp3)–C(sp3) Palladium-Catalyzed Cross-Coupling Read More »