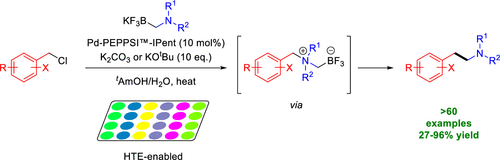
Research published in The Journal of Organic Chemistry, from Lippa, R. et al., has shown the access to substituted arylethylamines that are key structural components in natural, pharmaceutical, and agrochemical compounds. Substituted phenylethylamine and structurally related analogues represent a key pharmacophore found in endogenous and synthetic neurotransmitters, pharmaceutical agents, and agrochemical products including products such as dopamine, salbutamol, latrepirdine, fluopyram, selegiline and hordenine. This privileged class of compounds plays key pharmacological roles as central nervous system stimulants, nasal decongestants, antidepressants, anti-parkinson agents, and vasopressors among others. Due to the wide range of biological activities associated with this motif, the scaffold has remained an interesting and challenging problem to synthetic chemists.
This article describes the cross-coupling of N,N-dialkylaminomethyltrifluoroborate salts with various benzyl chlorides and chloromethyl-heteroaryls including 5-membered and 6-membered rings to obtain the desirable arylethylamine scaffold. Access to such scaffolds is achieved by palladium-catalyzed C(sp3)-C(sp3) coupling between (chloromethyl)aryls and air and moisture stable N,N-dialkylaminomethyl-trifluoroborate salts. A range of structurally and electronically varied arylethylamine products were obtained in moderate to excellent yields. This procedure utilizes stable, solid aminomethyltrifluoroborate salts and (chloromethyl)aryls, which can be sourced commercially or easily obtained from the corresponding alcohol. Conditions were initially identified optimized for the synthesis of (pyridin-3-yl)ethylamine derivatives and were found to be suitable for the selection of 5-membered heterocycles as well.
The Suzuki-Miyaura-type cross-coupling reaction with boronic acids and boronate esters has been widely studied and used as a synthetic method to prepare organic compounds of wide-ranging importance. Potassium trifluoroborate salts of boronic acids are versatile reagents in organic synthesis. They overcome some limitations of the electrophilic boron atom and can be used in the transition-metal-catalyzed reactions to introduce a wide range of groups including, alkyl, alkenyl, alkynyl, aryl, allyl, etc. The organotrifluoroborates are virtually crystalline solids or free flowing powders and robust on storage and their tetracoordinate stability keeps the oxidative homocoupling, protodeboronation to minimum via decomposition. This distinctive reactivity complements those of tricoordinate organoborons and permits nucleophiles to be utilized as reactants toward electrophilic functional groups incorporated within the organoboron compounds themselves.
N,N-dialkylaminomethyl-trifluoroborate salts also referred to as Molanderates from Frontier Specialty Chemicals as pioneered by Molander and coworkers, offer nontoxic air-/moisture-stable reagents for cross-coupling reactions. Such reagents, as starting material are readily available from Frontier Specialty Chemicals. Frontier Specialty Chemicals has extensive experience in the field of boronic acids and its derivatives. Frontier offered the first commercially available bis(pinacolato)diboron to the synthetic community. Plethora of such N,N-dialkylaminomethyl-trifluoroborates are readily available Frontier Specialty Chemicals. To mention few: Potassium N,N-diethylaminomethyltrifluoroborate (Catalog No. P10529), Potassium N,N-dimethylaminomethyltrifluoroborate (Catalog No. P10369), Potassium N-Boc-aminomethyltrifluoroborate (Catalog No. P13308), Potassium 1-trifluoroboratomethyl-4-(N-Boc)piperazine (Catalog No. P10329), Potassium 1-methyl-4-trifluoroboratomethylpiperazine (Catalog No. P10315), Potassium (morpholin-4-yl)methyltrifluoroborate (Catalog No. P10324), Potassium 1-trifluoroboratomethylpiperidine (Catalog No. P10328).