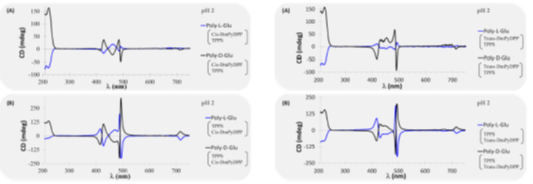
The Mammana group at the University of Dayton recently reported the self assembly of charged porphyrins onto a chiral substrate in the Journal Chirality. Charged water soluble porphyrins were used to produce chiral self-assembled structures by templating on to the chiral templates D or L polyglutamic acid. The negatively charged porphyrin, meso-Tetra(4-sulfonatophenyl)porphine cat. No. T1239, and the positively charged porphyrins, meso-trans-(di(N-methyl-4-pyridyl)diphenyl)porphine cat. No. D40923 and meso-cis-(di(N-methyl-4-pyridyl)diphenyl)porphine cat. No. D40922 were investigated. The induced chirality investigated by circular dichroism spectroscopy was found to depend on the pH of the solution, the chirality of the polyglutamic acid and the order of addition of the charged porphyrin species. meso-Tetra(4-sulfonatophenyl)porphine was found to undergo dynamic rearrangement of the supramolecular structure overtime, while meso-trans-(di(N-methyl-4-pyridyl)diphenyl)porphine interacts preferentially with random coils of the polyglutamate polymer, indicating potential use as a probe for protein secondary structure. Thanks for the use of our porphyrins in your research!