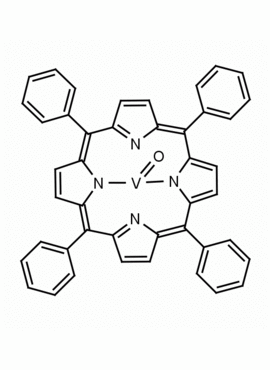
Vanadyl meso-tetraphenylporphine (1-3% chlorin) 5,10,15,20-Tetraphenyl-21H,23H-porphine vanadium(IV) oxide CAS: 14705-63-6 MDL: MFCD00012154
Molecular weight: 679.661 g/mol
Molecular Formula: C44H28N4OV
CAS Number: 14705-63-6
Storage: Store at room temperature, protect from light
Synonyms: 14705-63-6, MFCD00012154, Oxo[5,10,15,20-tetr
Field of Interest: Model compounds for vanadium containing crude oils, Epoxidation, Catalysis
Background: Vanadyl meso-tetraphenylporphine (1-3% chlorin) is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. Vanadyl meso-tetraphenylporphine has been used as a model for vanadium compounds in crude oil.1 Vanadyl meso-tetraphenylporphine is a selective catalyst for the epoxidation of olefins.2
References:
1.) Dechaine, et al. Chemistry and Association of Vanadium Compounds in Heavy Oil and Bitumen, and Implications for Their Selective Removal. Energy Fuels 2010, 24, 5, 2795–2808. https://doi.org/10.1021/ef100173j
2.) Maurya, et al. Selective epoxidation of olefins by vanadylporphyrin [VO(TPP)] and electron deficient nonplanar B-octabromovanadylporphyrin [VO(TPPBr8)]. Journal of Porphyrins and PhthalocyaninesVol. 26, No. 03, pp. 187-194 (2022).
3.) Taghavi, et al. Investigation of catalytic activity of high-valent vanadium(IV) tetraphenylporphyrin: A new, highly efficient and reusable catalyst for acetylation of alcohols and phenols with acetic anhydride. Inorganica Chimica Acta, 2011, vol. 377, # 1, p. 159 – 164. https://doi.org/10.1016/j.ica.2011.07.036
4.) Bai, et al. Cycloaddition of epoxide and CO2 to cyclic carbonate catalyzed by VO(IV) porphyrin. Applied Organometallic Chemistry, 2015, vol. 29, # 4, p. 240 – 243. https://doi.org/10.1002/aoc.3278
5.) Kobayashi, et al. Simple vanadium(V) catalyst for oxidation of alkane with O2 under mild conditions. Chemistry Letters, 2007, vol. 36, # 1, p. 114 – 115. https://doi.org/10.1246/cl.2007.114
6.) Maurya, et al. Selective Bromination of β-Positions of Porphyrin by Self-Catalytic Behaviour of VOTPP: Facile Synthesis, Electrochemical Redox Properties and Catalytic Application. European Journal of Inorganic Chemistry, 2021, vol. 2021, # 17, p. 1685 – 1694. https://doi.org/10.1002/ejic.202100116