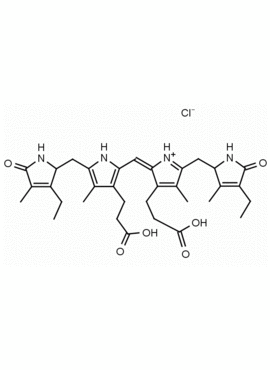
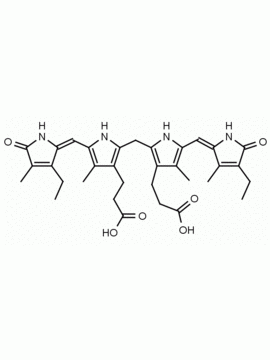
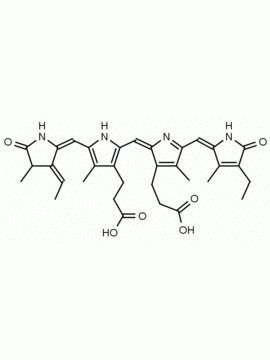
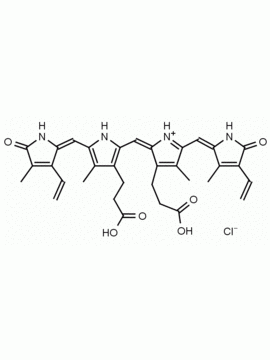
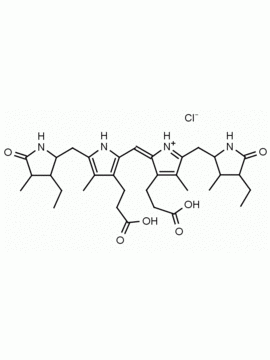
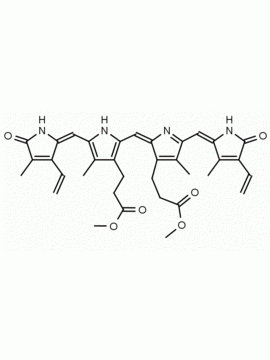
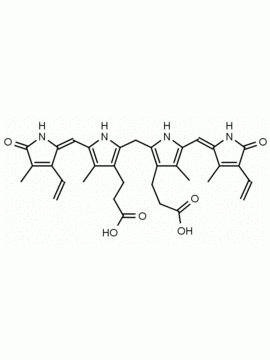
Urobilin hydrochloride CAS: 28925-89-5 MDL: MFCD00216796
Molecular weight: 627.17 g/mol
Molecular Formula: C33H42N4O6 HCl
CAS Number: 28925-89-5
Storage: Store at 0 – 4 °C, protect from light.
Synonyms: 28925-89-5, Urobilin IX hydroch
Field of Interest: Heme Catabolism and Phase II Metabolism
Background: Urobilin Hydrochloride is produced via the oxidation of urobilinogen, a degradation product of bilirubin, and excreted in the urine. Bilirubin is a water insoluble tetrapyrrole produced from the reduction of biliverdin in a reaction catalyzed by the enzyme bilirverdin reductase. Water insoluble bilirubin (also called indirect bilirubin) in vivo undergoes glucuronidation in the liver (addition of one or two glucuronic acids through a glycosidic bond) to form the water soluble bilirubin mono or diglucuronide (also called bilirubin conjugate or direct bilirubin). Bilirubin conjugate is excreted from the liver in bile or is converted to mesobilinogen via gut bacteria and then to urobilinogen and excreted in the urine as urobilin or stercobilinogen and excreted in the feces as stercobilin. Urobilin Hydrochloride is soluble in basic aqueous solutions (pH > 9 for intitial dissolution) and soluble down to pH 7 once in solution as well as methanol and ethanol if made slightly basic.1-4
References:
1.) Sampson, D. L., Y. L. Chng, et al. (2013). “The highly abundant urinary metabolite urobilin interferes with the bicinchoninic acid assay.” Analytical Biochemistry 442(1): 110.
2.) Quinn, K. D., N. Q. T. Nguyen, et al. (2012). “Tandem mass spectrometry of bilin tetrapyrroles by electrospray ionization and collision-induced dissociation.” Rapid Communications in Mass Spectrometry 26(16): 1767.
3.) Cuperus, F. J. C., A. M. Hafkamp, et al. (2009). “Effective Treatment of Unconjugated Hyperbilirubinemia With Oral Bile Salts in Gunn Rats.” Gastroenterology 136(2): 673.
4.) Mölzer, C., H. Huber, et al. (2012). “In vitro antioxidant capacity and antigenotoxic properties of protoporphyrin and structurally related tetrapyrroles.” Free Radical Research 46(11): 1369-1377.