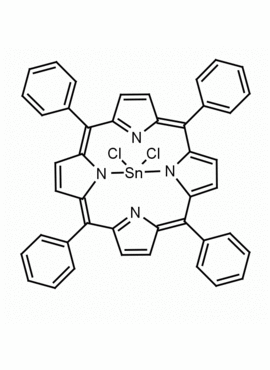
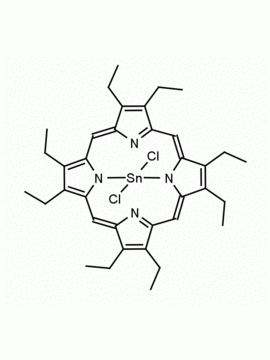
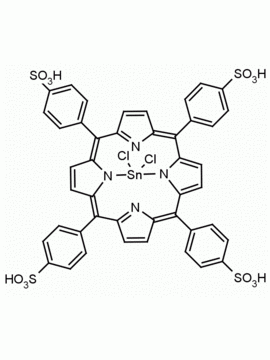
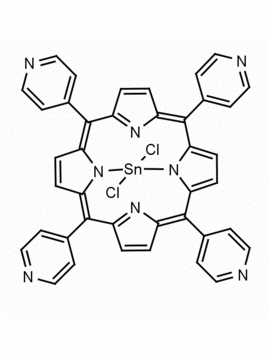
Sn(IV) meso-Tetraphenylporphine dichloride (contains 1-3% chlorin) CAS: 26334-85-0 MDL: MFCD00995672
Molecular weight: 802.34 g/mol
Molecular Formula: C44H28Cl2N4Sn
CAS Number: 26334-85-0
Storage: Store at room temperature, protect from light
Synonyms: Chlorure 5,10,15,20
Field of Interest: Catalysis, Metal-Organic Frameworks, Photophysics
Background: Sn(IV) meso-Tetraphenylporphine dichloride (contains 1-3% chlorin) is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Zhong, et al. Interfacial Self-Assembly Driven Formation of Hierarchically Structured Nanocrystals with Photocatalytic Activity. ACS Nano 2014, 8, 1, 827–833. https://doi.org/10.1021/nn405492d
2.) Baral, et al. Tin(IV)-Porphyrin Tetracarbonyl Cobaltate: An Efficient Catalyst for the Carbonylation of Epoxides. Catalysts 2019, 9(4), 311; https://doi.org/10.3390/catal9040311
3.) Johnson, et al. Porphyrin-Metalation-Mediated Tuning of Photoredox Catalytic Properties in Metal-Organic Frameworks. ACS Catalysis, 2015, vol. 5, # 9, p. 5283 – 5291. https://doi.org/10.1021/acscatal.5b00941
4.) Moghadam, et al. Rapid and efficient acetylation of alcohols and phenols with acetic anhydride catalyzed by electron-deficient tin(IV) porphyrin. Journal of Molecular Catalysis A: Chemical, 2004, vol. 219, # 1, p. 73 – 78. https://doi.org/10.1016/j.molcata.2007.05.012
5.) Slagt, et al. Supramolecular bidentate phosphorus ligands based on bis-zinc(ii) and bis-tin(iv) porphyrin building blocks. Dalton Transactions, 2007, # 22, p. 2302 – 2310. https://doi.org/10.1039/B702462M
6.) Arnold, et al. Tin porphyrins. 6. Tin-119 chemical shifts and line widths of tin(IV) complexes of tetraphenyl-, tetra-p-tolyl-, and octaethylporphyrin. Inorganic Chemistry, 1994, vol. 33, # 7, p. 1486 – 1490. https://doi.org/10.1021/ic00085a044
7.) Maiti, et al. Photophysics of soret-excited tetrapyrroles in solution. IV. Radiationless decay and triplet-triplet annihilation investigated using tetraphenylporphinato Sn(IV). Journal of Physical Chemistry A, 2009, vol. 113, # 42, p. 11318 – 11326. https://doi.org/10.1021/jp906966h
8.) Olsson, et al. Design of oxophilic metalloporphyrins: An experimental and DFT study of methanol binding. Dalton Transactions, 2018, vol. 47, # 33, p. 11572 – 11585. https://doi.org/10.1039/C8DT02432D