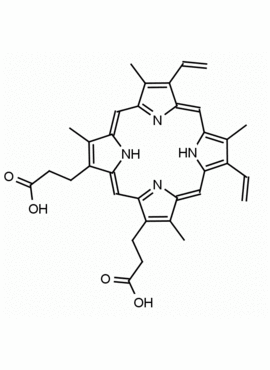
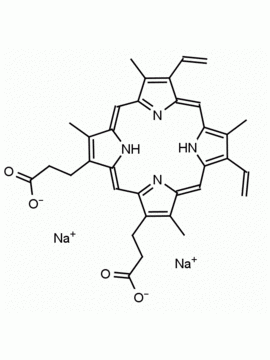
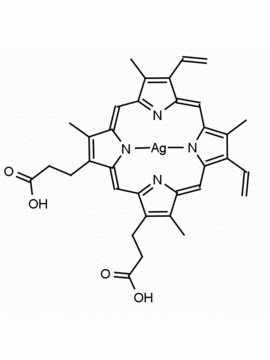
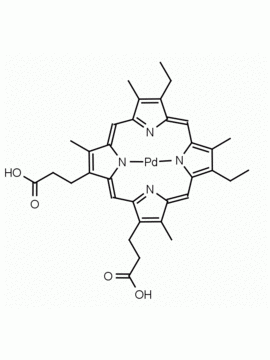
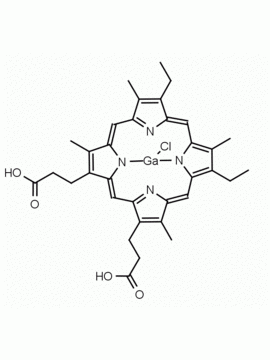
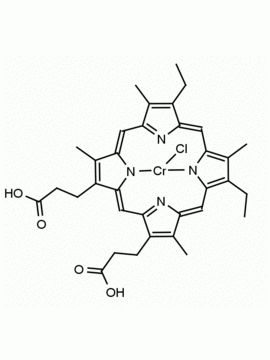
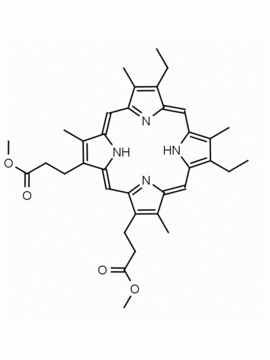
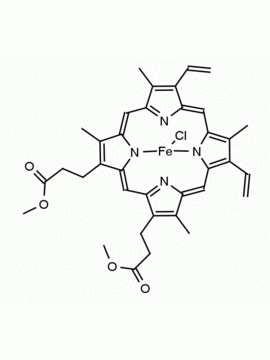
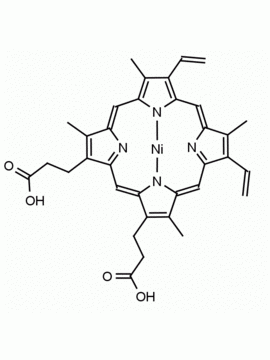
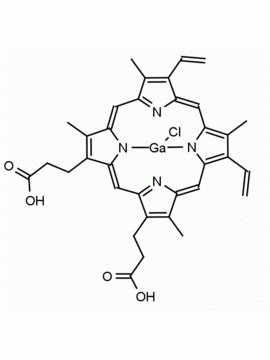
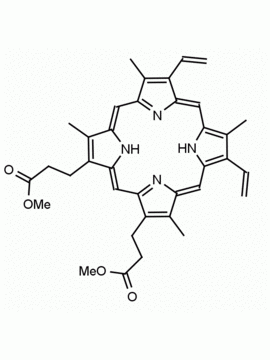
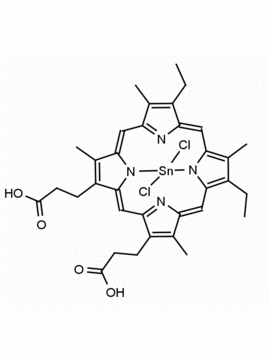
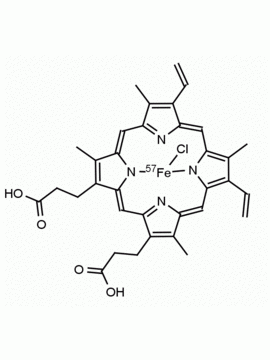
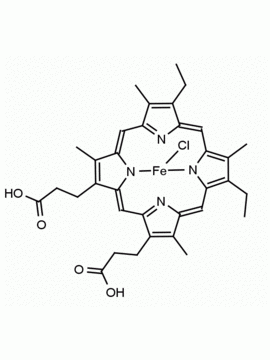
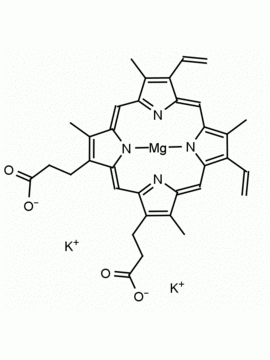
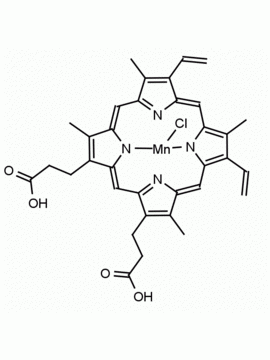
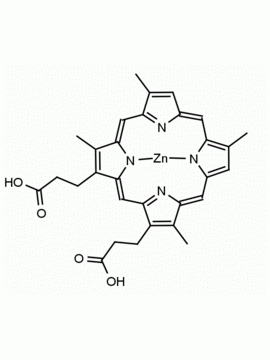
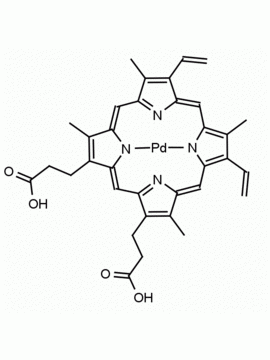
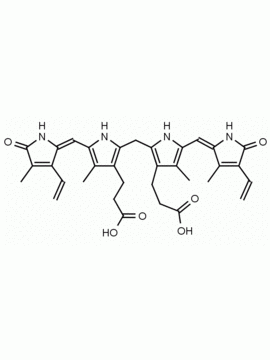
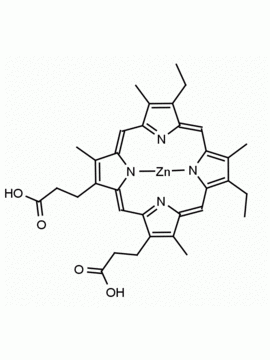
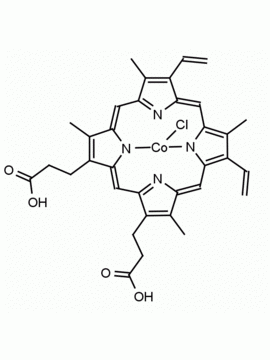
P562-9 Protoporphyrin IX 8,13-Bis(vinyl)-3,7,12,17-tetramethyl-21H,23H-porphine-2,18-dipropionic acid CAS: 553-12-8 MDL: MFCD00151109
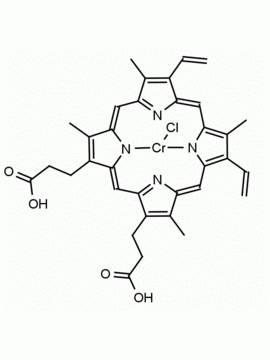
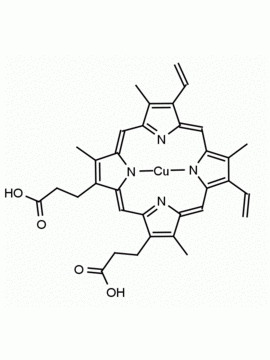
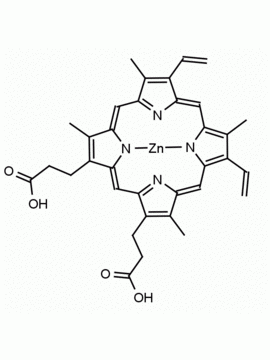
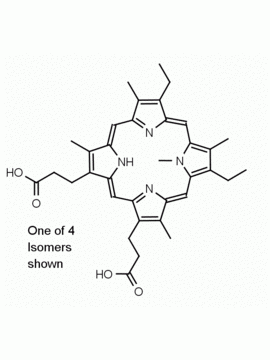
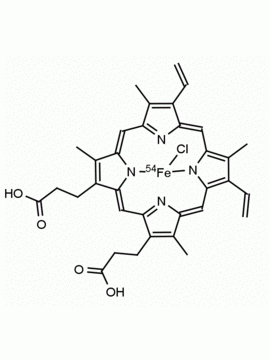
Background: Protoporphyrin IX is active as a photosensitizer and is a component of nitric oxide synthase for NOS generation.1, 2 Protoporphyrin IX is a substrate for heme oxygenases affording cellular protection and producing biliverdin and CO, a secondary messenger in cells, and metallated (Fe) versions of heme are one of the most abundant and widely used metalloporphyrins in the biosphere.3,4 Metallated protoporphyrin derivatives especially those containing tin and first row transition metals such as Zn, Mn, Cr, Ni are extensively used as experimental heme oxygenase inhibitors.5 Frontier Specialty Chemicals produces a wide range of metallated protoporphyrin derivatives for your experimental needs , including 54Fe and 57Fe protoporphyrin chlorides Gallium (III) protoporphyrin derivatives display potent photodynamic antimicrobial effects and is of current interest for the treatment of antibiotic resistant infections.6 Electrocatalytic reduction of CO2 to formic acid was demonstrated for various metallated protoporphyrins.7 Cobalt (III) protoporphyrin, when inserted into myoglobin serves as a catalyst for the reduction of N2O to N2.8 Co(III) and Sn(IV) protoporphyrin derivatives inactivate arboviruses including Chikungunya and Zika by targeting the viral envelope.9 Protoporphyrin is a potential biomarker for cancer screening.10 Aggregation behavior of protoporphyrin in water is complex and is dependent on pH and ionic strength.11 Magnesium (II) Protoporphyrin is the first intermediate in the biosynthesis of chlorophylls from protoporphyrin IX.12 Zinc (II) protoporphyrin IX is a potent competitive inhibitor of heme oxygenase and is formed naturally in the case of iron deficiency of lead poisoning.13 Copper (II) protoporphyrin IX does not inhibit heme oxygenase and is used as a negative control in inhibition studies. N-methyl protoporphyrin IX is a transition state analogue and a potent inhibitor of ferrochelatase, the enzyme that inserts iron into protoporphyrin IX and is commonly used to induce haem deficiency in cell cultures.14 Protoporphyrin is a reactive molecule and readily reacts with electrophilic reagents at the vinyl groups to produce new derivatives and is often an undesirable reaction pathway that is involved in its degradation in solution. Mesoporphyrin IX is very similar to protoporphyrin IX and is more stable for catalytic and material science applications that do not require the beta-vinyl groups.15
Molecular weight: 562.658 g/mol
Molecular Formula: C34H34N4O4
CAS Number: 553-12-8
Storage: Store at room temperature, protect from light.
Solubility: Protoporphyrin IX is soluble in polar organic solvents, especially pyridine. Dissolve initially in 0.1M base (Tris base or NaOH). Add a water miscible organic solvent (EtOH, MeOH, DMSO, DMF) until the solution is 50/50 base/solvent. When a clear solution is obtained, it can be diluted into an aqueous medium and titrated or buffered to any pH >7. Use immediately. Protoporphyrin IX slowly degrades in solution.
Synonyms: ChEMBL267548, 2,18-Porphinedipropionic acid, 3,8,13,17-tetramethyl-7,12-divinyl-, 21H,23H-Porphine-2,18-dipropanoic acid, 7,12-diethenyl-3,8,13,17-tetramethyl-, 3,3′-(3,7,12,17-Tetramethyl-8,13-divinylporphin-2,18-diyl)di(propionsaure), 3,3′-(3,7,12,17-tetramethyl-8,13-divinylporphine-2,18-diyl)di(propionic acid), Acide 3,3′-(3,7,12,17-tetramethyl-8,13-divinylporphine-2,18-diyl)dipropionique, acido 3,3′-(3,7,12,17-tetrametil-8,13-divinilporfina-2,18-diil)dipropionico, Kammerer’s porphyrin, NSC 177389, NSC 2632, Ooporphyrin, Protoporphyrin, Protoporphyrin IX
References:
1.) Castano, Ana P.; Demidova, Tatiana N.; Hamblin, Michael R., Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization, Photodiagnosis and Photodynamic Therapy (2005), Volume Date 2004, 1(4), 279-293.
2.) Alderton, Wendy K.; Cooper, Chris E.; Knowles, Richard G., Nitric oxide synthases: structure, function and inhibition, Biochemical Journal (2001), 357(3), 593-615.
3.) Gozzelino, Raffaella; Jeney, Viktoria; Soares, Miguel P., Mechanisms of cell protection by heme oxygenase-1, Annual Review of Pharmacology and Toxicology (2010), 50, 323-354.
4.) Poulos, Thomas L., Heme Enzyme Structure and Function, Chemical Reviews (Washington, DC, United States) (2014), 114(7), 3919-3962.
5.) Vreman, Hendrik. J.; Ekstrand, Bradley, C.; Stevenson, David, K., Selection of Metalloporphyrin Heme Oxygenase Inhibitors Based on Potency and Photoreactivity, Pediatric Research (1993), 33, 195-200.
6.) Visaggio, D.; Frangipani, E.; Hijazi, S.; Pirolo, M.; Leoni, L.; Rampioni, G.; Imperi, Francesco.; Bernstein, Lawrence.; Sorrentino, Raffaella.; Ungaro, Francesca.; Visca, Paolo., Variable Susceptibility to Gallium Compounds of Major Cystic Fibrosis Pathogens. ACS Infect. Dis. 2022, 8, 78-85.
7.) Fukuzumi, S.; Lee, Yong-Min.; Ahn, Hyun, S.; Nam, Wonwoo., Mechanisms of catalytic reduction of CO2 with heme and nonheme metal complexes. Chem. Sci., 2018, 9, 6017-6034.
8.) Rapson, T. D.; Warneke, S.; Musameh, M. M.; Dacres, H.; Macdonald, Ben, C.T.; Trowell, Stephen, C., Conversion of Nitrous Oxide to Nitrogen by Cobalt-Substituted Myoglobin. RSC Adv., 2-15, 5- 89003-89008.
9.) Neris, Romulo, L.S., et. al. Co-Protoporphyrin IX and Sn-protoporphyrin IX inactivate Zika, Chikungunya and other arboviruses by targeting the viral envelope. Scientific Reports., 8, 2018.
10.) Anna Walke, Eric Suero Molina, Walter Stummer and Simone König., Protoporphyrin IX Analysis from Blood and Serum in the context of neurosurgery of Glioblastoma. 10.5772/intechopen.95042.
11.) Scolaro, Luigi, M.; Castriciano, Mariangela.; Romeo, Andrea.; Patane, Salvatore.; Cefali, Eugenio, Allegrini, Maria., Aggregation Behavior of Protoporphyrin IX in Aqueous solutions: Clear Evidence of Vesicle Formation. J. Phys. Chem. B 2002, 106, 10, 2453-2459.
12.) Borah, Karishma, D.; Bhuyan, Jagannath., Magnesium porphyrins with relevance to chlorophylls. Dalton Trans., 2017, 46, 6497-6509.
13.) Kwok, Simon, C. M., Zinc Protoporphyrin Upregulates Heme Oxygenase-1 in PC-3 Cells via the Stress Response Pathway. International Journal of cell Biology, 2013.
14.) Shi, Z.; Ferreira, Gloria, C., Modulation of inhibition of ferrochelatase by N-methylprotoporphyrin. Biochem J., 2006, 399, 21-28.
15.) Sitte, Elisabeth.; Senge, Mathias, O., The Red Color of Life Transformed – Synthetic Advances and Emerging Applications of Protoporphyrin IX in Chemical Biology. Eur. J. Org. Chem. 2020, 3171-3191.
UV-Visible Spectrum of Protoporphyrin IX: