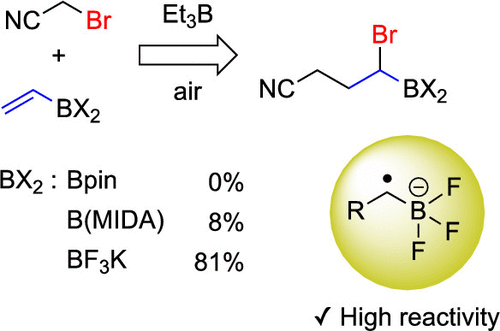
Research from Ueda, M. et al., group at Osaka Prefecture University published in Organic Letters has shown that the α-boryl radical of potassium alkyltrifluoroborates have higher reactivity compared to their corresponding alkylboronic acid pinacol esters and alkyl N-methyl imidodiacetic acid (MIDA) boronates in the halogen atom abstraction step of atom-transfer radical addition (ATRA) between alkyl bromide and vinylborons. This study of an ATRA of alkyl halides with potassium vinyltrifluoroborate (Catalog No. P10254) and potassium isopropenyltrifluoroborate (Catalog No. P10407) has enabled the synthesis of unique alkyborons.