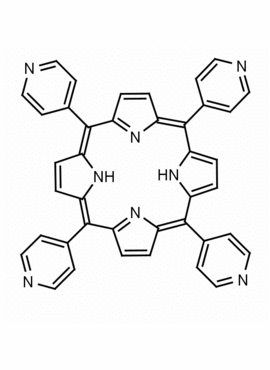
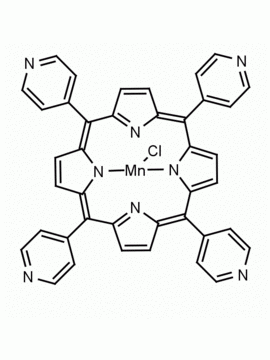
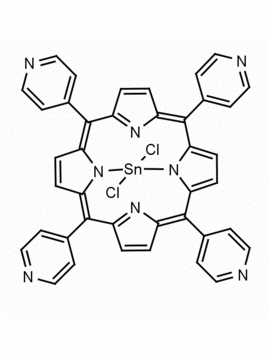
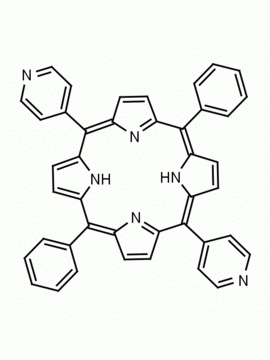
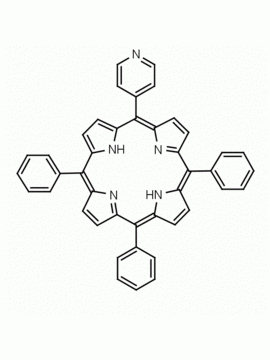
meso-Tetra(4-pyridyl)porphine 5,10,15,20-Tetra(4-pyridyl)-21H,23H-porphine CAS: 16834-13-2 MDL: MFCD00009626
Molecular weight: 618.7 g/mol
Molecular Formula: C40H26N8
CAS Number: 16834-13-2
Storage: Store at room temperature, protect from light
Synonyms: 5,10,15,20-Tetra(4-pyridyl)-21H,23H-porphine, 5,10,15,20-Tetra(4-pyridyl)porphyrin, meso-Tetra(4-pyridyl)porphine, CHEMBL4084530, ACMC-209dy0,, tetrakis(4-pyridyl)porphyrin, meso-Tetra-4-pyridylporphine, SCHEMBL245937
Fields of Interest: Photodynamic Therapy, MOFs, Nano Assemblies, Photophysics, Sensors
Background: Meso tetra(4-pyridyl)porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. Meso tetra(4-pyridyl)porphine is useful for the construction of supramolecular complexes, MOFs, coordination polymers , thin films and sensors.
Selected References:
1.) Akins DL, Zhu H-R, Guo C. 1996. Aggregation of Tetraaryl-Substituted Porphyrins in Homogeneous Solution. Phys. Chem. 100:5420-5
2.) Anderson S, Anderson HL, Bashall A, McPartlin M, Sanders JKM. 1995. Assembly and crystal structure of a photoactive array of five porphyrins. Chem., Int. Ed. Engl. 34:1096-9
3.) Auwarter W, Weber-Bargioni A, Brink S, Riemann A, Schiffrin A, et al. 2007. Controlled metalation of self-assembled porphyrin nanoarrays in two dimensions. ChemPhysChem 8:250-
4.) Bernadou J, Pratviel G, Bennis F, Girardet M, Meunier B. 1989. Potassium monopersulfate and a water-soluble manganese porphyrin complex, [Mn(TMPyP)](OAc)5, as an efficient reagent for the oxidative cleavage of DNA. Biochemistry 28:7268-75
4.)Caughey WS, Raymond LD, Horiuchi M, Caughey B. 1998. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Natl. Acad. Sci. U. S. A. 95:12117-22
6.) Drain CM, Nifiatis F, Vasenko A, Batteas JD. 1998. Porphyrin tessellation by design: metal-mediated self-assembly of large arrays and tapes. Chem., Int. Ed. 37:2344-7
7.) Fleischer EB, Shachter AM. 1991. Coordination oligomers and a coordination polymer of zinc tetraarylporphyrins. Chem. 30:3763-9
8.)Gong X, Milic T, Xu C, Batteas JD, Drain CM. 2002. Preparation and Characterization of Porphyrin Nanoparticles. Am. Chem. Soc. 124:14290-1
9.)Hagrman D, Hagrman PJ, Zubieta J. 1999. Solid-state coordination chemistry: the self-assembly of microporous organic-inorganic hybrid frameworks constructed from tetrapyridylporphyrin and bimetallic oxide chains or oxide clusters. Chem., Int. Ed. 38:3165-8
10.) Ishii H, Seki K. 1997. Energy level alignment at organic/metal interfaces studied by UV photoemission: breakdown of traditional assumption of a common vacuum level at the interface. IEEE Trans. Electron Devices 44:1295-301
11.) Li D, Swanson BI, Robinson JM, Hoffbauer MA. 1993. Porphyrin based self-assembled monolayer thin films: synthesis and characterization. Am. Chem. Soc. 115:6975-80
12.) Liu K, Liu Y, Yao Y, Yuan H, Wang S, et al. 2013. Supramolecular Photosensitizers with Enhanced Antibacterial Efficiency. Chem., Int. Ed. 52:8285-9
13.) Sari MA, Battioni JP, Dupre D, Mansuy D, Le Pecq JB. 1990. Interaction of cationic porphyrins with DNA: importance of the number and position of the charges and minimum structural requirements for intercalation. Biochemistry 29:4205-15
14.) Schmitt F, Govindaswamy P, Suess-Fink G, Ang WH, Dyson PJ, et al. 2008. Ruthenium Porphyrin Compounds for Photodynamic Therapy of Cancer. Med. Chem. 51:1811-6
15.) Sharma CVK, Broker GA, Huddleston JG, Baldwin JW, Metzger RM, Rogers RD. 1999. Design Strategies for Solid-State Supramolecular Arrays Containing Both Mixed-Metalated and Freebase Porphyrins. Am. Chem. Soc. 121:1137-44
16.) Shi D-F, Wheelhouse RT, Sun D, Hurley LH. 2001. Quadruplex-Interactive Agents as Telomerase Inhibitors: Synthesis of Porphyrins and Structure-Activity Relationship for the Inhibition of Telomerase. Med. Chem. 44:4509-23
17.) Slone RV, Hupp JT. 1997. Synthesis, Characterization, and Preliminary Host-Guest Binding Studies of Porphyrinic Molecular Squares Featuring fac-Tricarbonylrhenium(I) Chloro Corners. Chem. 36:5422-3
18.) Wang Z, Medforth CJ, Shelnutt JA. 2004. Porphyrin Nanotubes by Ionic Self-Assembly. Am. Chem. Soc. 126:15954-5
19.) Xing B, Choi M-F, Xu B. 2002. Design of coordination polymer gels as stable catalytic systems. – Eur. J. 8:5028-32
20.) Zheng Y-R, Zhao Z-G, Wang M, Ghosh K, Pollock JB, et al. 2010. A Facile Approach toward Multicomponent Supramolecular Structures: Selective Self-Assembly via Charge Separation. J. Am. Chem. Soc. 132:16873-82
21.) Muthukumar, et al. Highly sensitive detection of HCl gas using a thin film of meso-tetra(4-pyridyl)porphyrin coated glass slide by optochemical method. Sensors and Actuators B: Chemical. Volume 159, Issue 1, 28 November 2011, Pages 238-244.