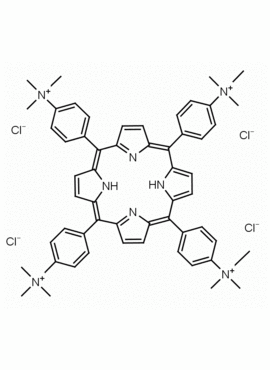
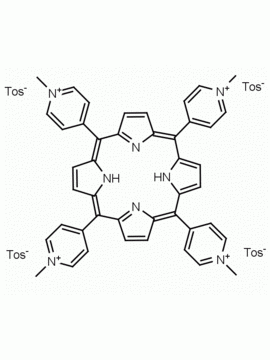
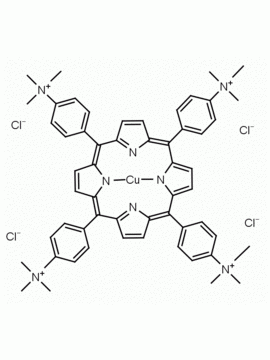
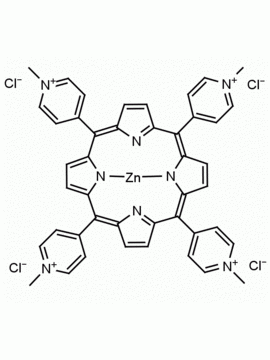
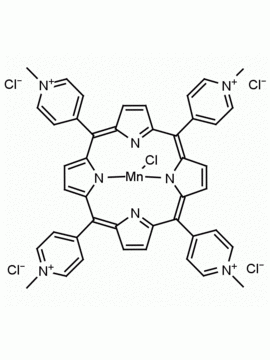
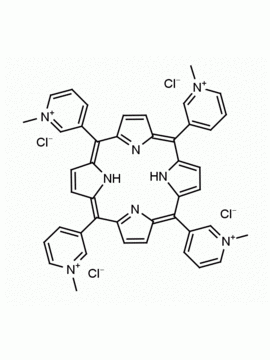
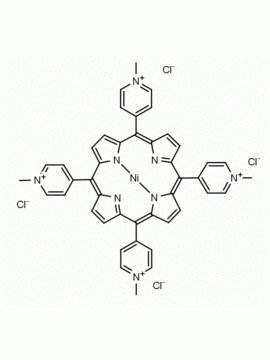
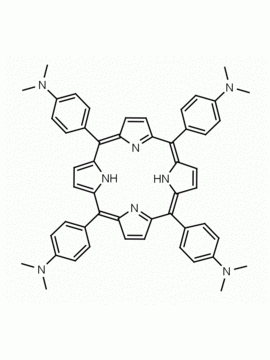
meso-Tetra(4-N,N,N-trimethylanilinium) porphine tetrachloride 5,10,15,20-Tetrakis(4-trimethylammoniophenyl) porphine tetrachloride CAS: 92739-64-5 MDL: MFCD03425989
Molecular weight: 988.9 g/mol
Molecular Formula: C56H62Cl4N8
CAS Number: 92739-64-5
Storage: Store at room temperature, protect from light.
Synonyms: MESO-TETRA(4-N,N,N-TRIMETHYLANILINIUM) PORPHINE TETRACHLORIDE,92739-64-5, CHEMBL4575600,tetrakis(4-trimethylammoniophenyl) porphyrin tetra-chloride, MESO-TETRA(4-N,N,N-TRIMETHYLANILINIUM)PORPHINETETRACHLORIDE
Field of Interest: Water Soluble Porphyrins, Photodynamic Therapy, Hydrogen Storage, Photoinactivation, Oligonucleotide Delivery
Background: meso-Tetra(4-N,N,N-trimethylanilinium) porphine tetrachloride is active as a photodynamic therapy agent against Murine LM3 tumor1. Meso-Tetra (4-N,N,N-Trimethylanilinium) porphine.has been used as a probe in the beta-hematin assay for antimalarial drug action2. Meso-Tetra (4-N,N,N-Trimethylanilinium) porphine aggregates with anionic porphyrins where found to possess hydrogen storage potential in the solid state3. Cationic porphyrins such as meso-Tetra (4-N,N,N-Trimethylanilinium) porphine have been used for fluorescent DNA counterstaining in conjugation with immunofluorescence4. Meso-Tetra (4-N,N,N-Trimethylanilinium) porphine effectively inactivate Gram-negative bacteria by photoinactivation5. Water soluble cationic porphyrins such as Meso-Tetra (4-N,N,N-Trimethylanilinium) porphine show efficacy for the cellular delivery of oligonucleotides6.
References:
1.) Colombo, L.L, Juarranz, A., Canete, M., Villanueva, A., Stockert, J.C.; Photodynamic Therapy of the Murine LM3 Tumor Using Meso-Tetra (4-N,N,N-Trimethylanilinium) porphine. Int J Biomed Sci.2007, 3, 258-262. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3614653/
2.) Ziegler, J., Pasierb, L., Cole, K.A., Wright, D.W.; Metalloporphyrin probes for antimalarial drug action. J Inorg Biochem2003, 96, 478-486. doi: 10.1016/s0162-0134(03)00253-8
3.) Oztek, M.T.; Hampton, M.D., Slattery, D.K., Louks, S.; Hydrogen storage with hetero porphyrin aggregates. International Journal of Hydrogen Energy 2011,36, 6705-6710. https://doi.org/10.1016/j.ijhydene.2011.02.065
4.) Juarranz, A., Villanueva, A., Canete, M., Stockert,J.C.; Fluorescent porphyrin counterstaining of chromatin DNA in conjugation with immunofluorescence methods using FITC-labelled antibodies. Journal of Microscopy1996,182, 46-49. https://doi.org/10.1046/j.1365-2818.1996.114394.x
5.) Merchat, M., Bertolini, G., Giacomini, P., Villaneuva, A., Jori, G.; Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. Journal of Photochemistry and Photobiology B: Biology 1996, 32, 153-157. https://doi.org/10.1016/1011-1344(95)07147-4
6.) Flynn, S.M., George, S.T., White, L., Devonish, E., Takle, G.B.; Water-Soluble, Meso-Substituted Cationic Porphyrins-a Family of Compounds for Cellular Delivery of Oligonucleotides. BioTechniques1999, 26, 736-746. doi: 10.2144/99264rr03.