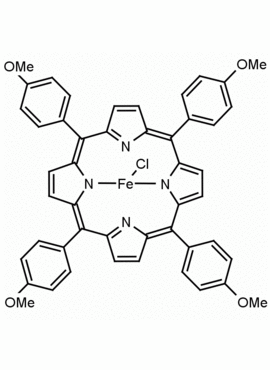
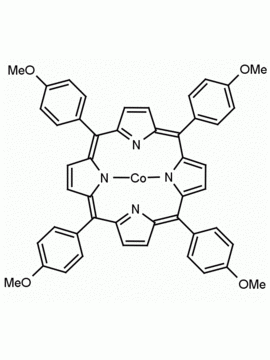
Fe(III) meso-Tetra(4-methoxyphenyl) porphine chloride CAS: 36995-20-7 MDL: MFCD00012405
Molecular weight: 824.12 g/mol
Molecular Formula: C48H36ClFeN4O4
CAS Number: 36995-20-7
Storage: Store at room temperature, protect from light
Synonyms: 36995-20-7, Chlor[5,10,15,20-te
Fields of Interest: Synthetic Porphyrins, Iron Porphyrins, Catalysis, Cyclopropanation, Proton Coupled Electron Transfer (PCET)
Background: Fe(III) meso-Tetra(4-methoxyphenyl) porphine chloride is a synthetic porphyrin derivative manufactured by Frontier Specialty Chemicals. Fe(III) meso-Tetra(4-methoxyphenyl) porphine chloride is useful for numerous catalytic transformations of organic and small molecules.1-6
References:
1.) Wolf, et al. Shape and stereoselective cyclopropanation of alkenes catalyzed by iron porphyrins. J. Am. Chem. Soc. 1995, 117, 36, 9194–9199. https://doi.org/10.1021/ja00141a011
2.) Baumann, et al. Iron Porphyrin Catalyzed N−H Insertion Reactions with Ethyl Diazoacetate. Organometallics 2007, 26, 16, 3995–4002. https://doi.org/10.1021/om0610997
3.) Guo, et al. A new evidence of the high-valent oxo–metal radical cation intermediate and hydrogen radical abstract mechanism in hydrocarbon hydroxylation catalyzed by metalloporphyrins. Journal of Molecular Catalysis A: Chemical. Volume 157, Issues 1–2, 20 June 2000, Pages 31-40. https://doi.org/10.1016/S1381-1169(99)00444-6
4.) Wang, et al. A Green Process for Oxidation of p-Nitrotoluene Catalyzed by Metalloporphyrins under Mild Conditions. Org. Process Res. Dev. 2006, 10, 4, 757–761. https://doi.org/10.1021/op060056z
5.) Gericke, et al. Oxo-Free Hydrocarbon Oxidation by an Iron(III)-Isoporphyrin Complex. Inorg. Chem. 2020, 59, 19, 13952–13961. https://doi.org/10.1021/acs.inorgchem.0c01618
6.) Lu, et al. Dioxygen reduction catalyzed by substituted iron tetraphenylporphyrins in acidic media. Journal of Porphyrins and Phthalocyanines. Vol. 16, No. 03, pp. 310-315 (2012). https://doi.org/10.1142/S1088424612500356