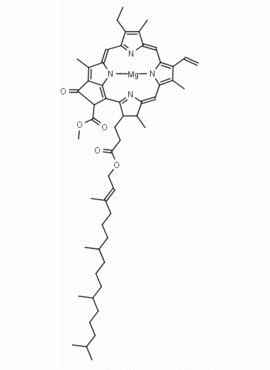
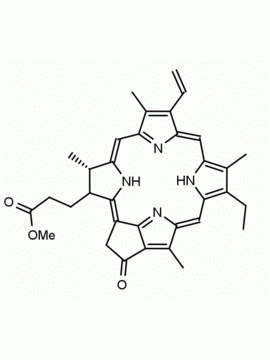
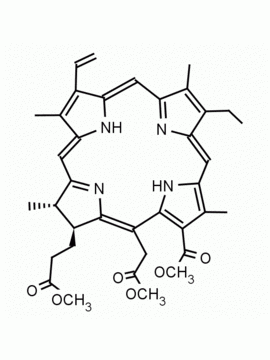
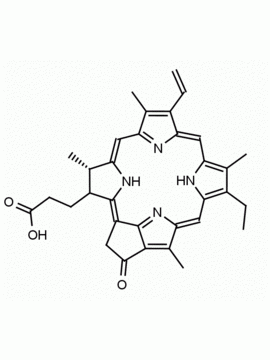
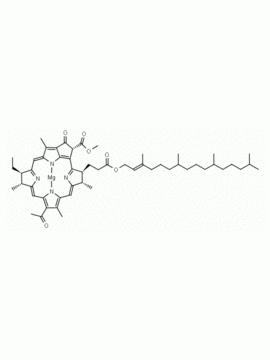
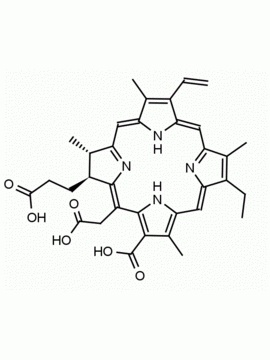
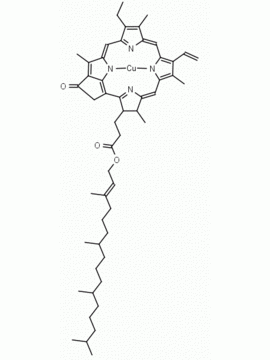
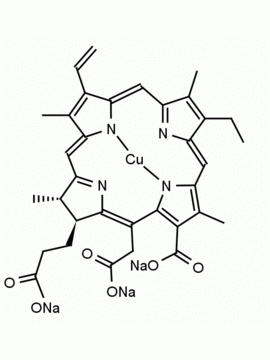
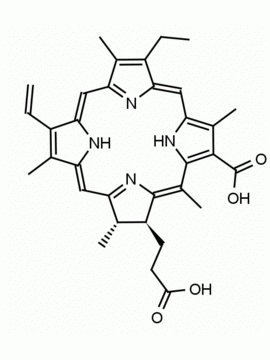
Chlorophyll a CAS: 479-61-8 MDL:
Alternative Names:
[(1Z,3R,9Z,14Z,18Z,5,8 10,13 15,18 2,6 2 2 5,8 .110,13 .115,18 2,6 2 2 [ACD/IUPAC Name]
[Methyl (3S,4S,21R)2 2 5,8 .110,13 .115,18 2,6 2 2 Chlorophyll a, Chlorophyll-1, Magnesium pheophytin, Magnesium, [(3S,4S, 21R)-9-ethenyl-14-e thyl-21-(methoxycar bonyl)-4,8,13,18-te tramethyl-20-oxo-3- phorbinepropanoato( 2-)-κN 23 ,κN 25 , (2E,7R,11R)-3,7,1 1,15-tetramethyl-2- hexadecen-1-yl este r],
Vegetable chlorophy(SP-4-2)-((2E,7R,11 [(2E,7R,11R)-3,7,11 1208847, 207-536-6, 4651978, deodophyll
Molecular Formula: C55 H72 MgN4 O5
MW: 893.489 CAS: 479-61-8
MFCD:
Storage: Store at -20 °C, protect from light.
Purity: > 95%
Field of Interest: Natural Product
Background: Frontier Specialty Chemicals provides high purity chlorophyll a for your research and development applications. Chlorophyll a is the main photosynthetic pigment of green plants and participates in light harvesting and the conversion of absorbed photons into chemical energy.1 Singlet and triplet state properties of chlorophylls and bacteriochlorphylls were measured in nucleophilic solvents.2 The crystal structure of ethyl chlorophyllide a has been determined.3
References:
1.) Bjorn. et al. A viewpoint: Why chlorophyll a ? Photosynthesis Research volume 99pages 85–98 (2009 ).
2.) Needwiedzki, et. al. Singlet and triplet excited state properties of natural chlorophylls and bacteriochlorophylls . Photosynthesis Research volume 106pages 227–238 (2010 ).
3.) Chow, et al. Crystal and molecular structure and absolute configuration of ethyl chlorophyllide a-dihydrate. Model for the different spectral forms of chlorophyll a . J. Am. Chem. Soc. 1975 , 97 , 25
3.) Aminot, A., and Rey, F. (2001) Chlorophyll a: Determination by spectroscopic methods . ICES Techniqnes in Marine Environmental Sciences, No. 30, 17pp. DOI: http://dx.doi.org/10.25607/OBP-278
4.) Maxwell, et al. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany , Volume 51, Issue 345, April 2000, Pages 659–668, https://doi.org/10.1093/jexbot/51.345.659
5.) Wellburn, The Spectral Determination of Chlorophylls a and b , as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution . Journal of Plant Physiology. Volume 144, Issue 3 , September 1994, Pages 307-313. https://doi.org/10.1016/S0176-1617(11)81192-2
SDS