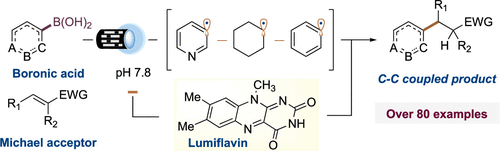
Steven Bloom group present in ACS Catalysis a general catalytic platform to access diverse heteroaromatic radicals, aromatic and aliphatic radicals from commercial boronic acids using a cofactor-derived flavin photocatalyst in aqueous media. They showed the capability of these radicals to engage Michael acceptors via single-electron conjugate addition. In this method they demonstrated that water acts as a key solvent and possible reagent for radical generation and open-shell alkylation. Also, they presented an aqueous protocol that help transform aliphatic, aromatic, and heteroaromatic boronic acids to C-centered radicals using a bioinspired flavin photocatalyst. Thank you for using our products. 6-Fluoro-5-methoxypyridin-3-ylboronic acid F14674, 2-Methoxypyridin-4-boronic acid M1210, 2-Methoxypyridine-5-boronic acid M5399, 3-Fluoro-2-methoxypyridine-4-boronic acid F13582, Quinoline-5-boronic acid Q1089, Quinoline-6-boronic acid B13020, Isoquinoline-6-boronic acid I1317, Dibenzofuran-4-boronic acid D5438, Thiophene-3-boronic acid JK110927.