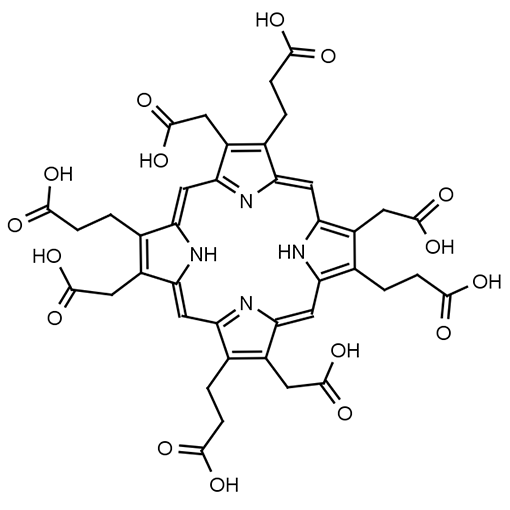
Name: Uroporphyrin I
See related porphyria research compounds:
Molecular Formula: C40H38N4O16
CAS#: 607-14-7
SMILES: OC(=O)CCC1=C(CC(O)=O)/C2=C/C3=N/C(=CC4=C(CCC(O)=O)C(CC(O)=O)=C(N4)/C=C4N=C(C=C1N2)C(CC(O)=O)=C4CCC(O)=O)/C(CC(O)=O)=C3CCC(O)=O
MDL#: MFCD00058110
Catalog#: URO1
Molecular weight: 830.474 g/mol
Other names:
- Uroporphyrin 1
Fields of Interest: Uroporphyrin I is an important analyte used in the diagnosis of porphyria diseases. Uroporphyrin I is the oxidized porphyrin form of uroporphyrinogen I. The presence of Uroporphyrin I (or uroporphyrinogen I) in biological samples indicates a malfunction of the heme biosynthesis pathway in disorders such as congenital erythropoietic porphyria. Uroporphyrin I has been used as an MRI contrast agent, as a fluorescent marker, and in photodynamic therapy. Researchers are also exploring its accumulation effects in tissues, potential as a biomarker for environmental toxin exposure, and its implications in cancer detection. Its fluorescent properties make it useful in imaging studies and photodynamic therapy research, particularly in targeting abnormal cells. Additionally, Uroporphyrin I’s interactions with metal ions and role in oxidative processes are being examined to better understand its contribution to cellular damage and aging mechanisms.
Background & Applications: Uroporphyrin I is a porphyrin that occurs in nature. This is an important analyte used in the determination of porphyria diseases. High levels of Uroporphyrin I indicate that the enzyme to convert Hydroxymethylbilane to Uroporphyrin III is deficient. In the absence of Uroporphyrin Synthase, hydroxymethylbilane spontaneously forms Uroporphyrinogen I (which is oxidized to form Uroporphyrin I).
Appearance: Very Dark Red to Black solid
Purity: >95% Uroporphyrin, >90% Uroporphyrin I
Storage: -20 °C
Solubility: Uroporphyrin I is soluble in basic aqueous media (pH >9.5) or in highly acidic media (pH <2). Beware that when dissolved in highly acidic aqueous solutions there may be some degradation of the uroporphyrin to form decarboxylated byproducts.
Literature:
- Harada, Y.; Murayama, Y.; Takamatsu, T.; Otsuji, E.; Tanaka, H. 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence Imaging for Tumor Detection: Recent Advances and Challenges. Int. J. Mol. Sci. 2022, 23, 6478. https://doi.org/10.3390/ijms23126478
- Kiening, M.; Lange, N. A Recap of Heme Metabolism towards Understanding Protoporphyrin IX Selectivity in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7974. https://doi.org/10.3390/ijms23147974
- Di Pierro, E.; De Canio, M.; Mercadante, R.; Savino, M.; Granata, F.; Tavazzi, D.; Nicolli, A.M.; Trevisan, A.; Marchini, S.; Fustinoni, S. Laboratory Diagnosis of Porphyria. Diagnostics 2021, 11, 1343. https://doi.org/10.3390/diagnostics11081343
- Phillips, J. D. Heme Biosynthesis and the Porphyrias Molecular Genetics and Metabolism 2019, 3, 164-177. https://doi.org/10.1016/j.ymgme.2019.04.008