U590-9 Urobilin HCl Technical Data Sheet
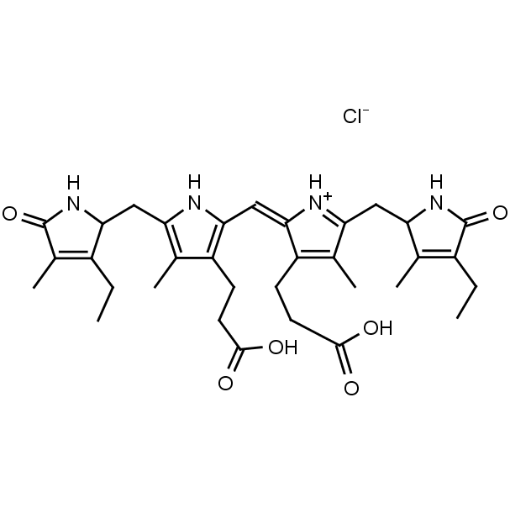
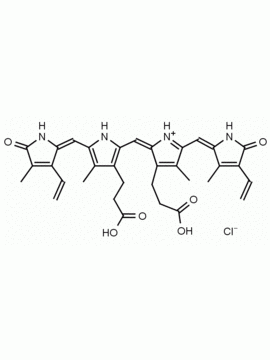
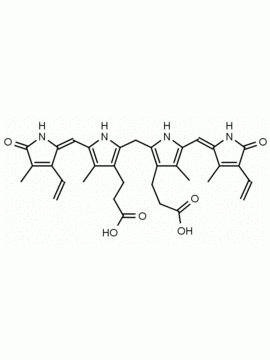
Name: Urobilin hydrochloride
Molecular Formula: C33H43ClN4O6
CAS#: 28925-89-5
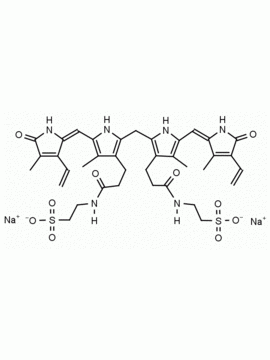
SMILES: [Cl-].CCC1=C(C)C(=O)NC1CC1=C(C)C(CCC(O)=O)=C(N1)C=C1[NH+]=C(CC2NC(=O)C(CC)=C2C)C(C)=C1CCC(O)=O
MDL#: MFCD00216796
Catalog#: U590-9
Molecular weight: 627-171
Fields of Interest: Used in elucidation metabolic pathways for degradation and elimination of heme (see more about heme biosynthesis)
Background & Applications: Urobilin Hydrochloride is produced via the oxidation of urobilinogen, a degradation product of bilirubin, and excreted in the urine. Bilirubin is a water insoluble tetrapyrrole produced from the reduction of biliverdin in a reaction catalyzed by the enzyme bilirverdin reductase. Water insoluble bilirubin (also called indirect bilirubin) in vivo undergoes glucuronidation in the liver (addition of one or two glucuronic acids through a glycosidic bond) to form the water-soluble bilirubin mono or diglucuronide (also called bilirubin conjugate or direct bilirubin). Bilirubin conjugate is excreted from the liver in bile or is converted to mesobilinogen via gut bacteria and then to urobilinogen and excreted in the urine as urobilin or stercobilinogen and excreted in the feces as stercobilin. Urobilin hydrochloride is soluble in basic aqueous solutions (pH > 9 for initial dissolution) and soluble down to pH 7 once in solution, as well as methanol and ethanol if made slightly basic.1-4
Purity: >95% (combined (E) and (Z) isomers)
Storage: 0-5 °C
Literature:
4.) Heirwegh, K. P. M. (1982). Bilirubin: Volume I: Chemistry. CRC Press.