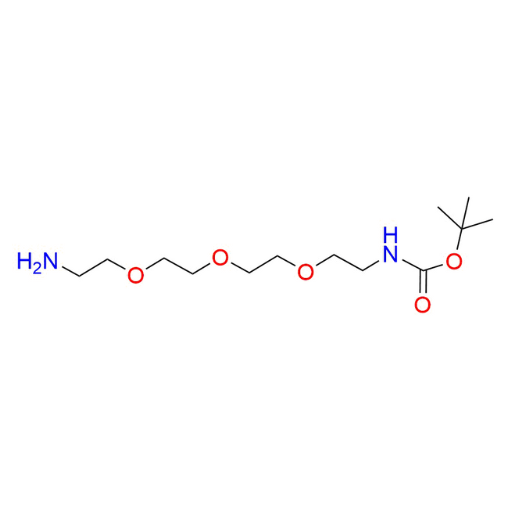
Name: tert-Butyl (2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)ethyl)carbamate
Molecular Formula: C13H28N2O5
CAS#: 101187-40-0
SMILES: NCCOCCOCCOCCNC(OC(C)(C)C)=O
MDL#: MFCD16619220
Catalog#: AMTGC226-BC16
Molecular weight: 292.37 g/mol
Other names:
- 1-Boc-amine-11-amino-3,6,9-trioxaundecane
Fields of Interest: PEGylation, bioconjugation, drug delivery, materials science
Background & Applications:
Background
tert-Butyl (2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)ethyl)carbamate (CAS 101187-40-0) is a protected amine-functionalized PEG derivative based on a PEG4 backbone, featuring a terminal Boc-protected primary amine and a free primary amine at the opposite end. The polyethylene glycol spacer provides hydrophilicity, flexibility, and compatibility with aqueous and biological environments, while the tert-butoxycarbonyl (Boc) group enables controlled, stepwise synthetic strategies by temporarily masking one amine functionality. The Boc protecting group can be readily removed under mild acidic conditions to generate a diamine PEG linker. This compound serves as a versatile intermediate within a robust portfolio of functionalized PEGs designed for precise molecular assembly.
Applications
This Boc-protected diamine PEG linker is commonly used in bioconjugation, drug delivery, and materials science applications requiring orthogonal reactivity and staged functional group exposure. Typical uses include selective PEGylation of small molecules, peptides, or polymers, stepwise linker construction, and preparation of multifunctional intermediates for pharmaceutical and biomaterials research. Following deprotection, the resulting diamine PEG is suitable for direct coupling to activated carboxylic acids, NHS esters, and isocyanates. As part of a comprehensive functionalized PEG product line, tert-Butyl (2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)ethyl)carbamate supports modular design strategies for advanced chemical and biological applications.
Appearance: Pale yellow, viscous liquid
Purity: 98%
Storage: 0-3 °C for long term storage
Solubility: DCM, Chloroform, MeOH
Literature:
- Tetrahedron Letters, 1998, vol. 39, # 48, p. 8799 – 8802
- Bulletin of the Chemical Society of Japan, 2019, vol. 92, # 5, p. 995 – 1000