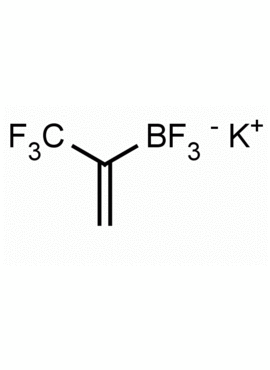
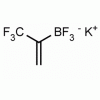
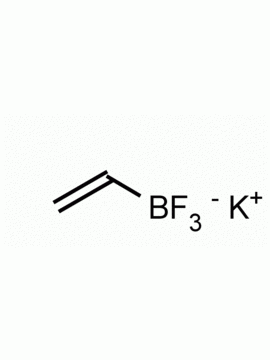
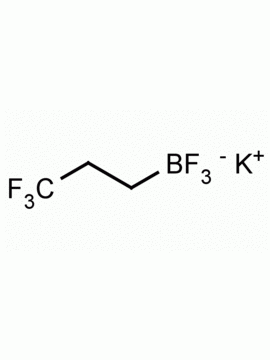
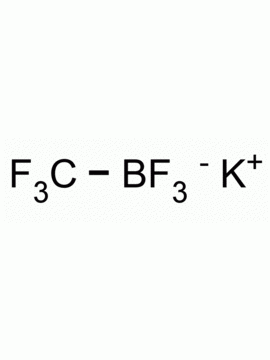Potassium trifluoro(3,3,3-trifluoroprop-1-en-2-yl)borate CAS: 2144763-11-9 MDL: MFCD28954621
Sizes Available: 250 mg, 1 g, and larger sizes available
Molecular Weight: 147.98 g/mol
Molecular Formula: C3H5BF3K
CAS Number: 2144763-11-9
Trifluoroborate Derivative Coupling Reactions
1.) Modern synthetic methods for copper-mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S bond formation, Ley, Steven V., Thomas, Andrew W., Angewandte Chemie, International Edition (2003), 42(44), 5400-5449.
2.) Suzuki-Miyaura cross-coupling reactions of alkylboronic acid derivatives or alkyitrifluoroborates with aryl, alkenyl or alkyl halides and triflates, Doucet, Henri, European Journal of Organic Chemistry (2008), (12), 2013-2030. DOI:10.1002/ejoc.200700984
3.) Palladium-catalyzed, direct boronic acid synthesis from aryl chlorides: a simplified route to diverse boronate ester derivatives, Molander, Gary A., Trice, Sarah L. J., Dreher, Spencer D., J. A. C.S. (2010), 132(50), 17701-17703.
4.) Scope of the Suzuki-Miyaura Cross-Coupling Reactions of Potassium Heteroaryltrifluoroborates. Molander, Gary A., Canturk, Belgin, Kennedy, Lauren E., J. of Organic Chemistry (2009), 74(3), 973-980.
5.) Enantioselective Organo-SOMO Catalysis: The α-Vinylation of Aldehydes, Kim, Hahn, MacMillan, David W. C., Journal of the American Chemical Society (2008), 130(2), 398-399. DOI:10.1021/ja077212h
6.) Visible-Light-Induced Chemoselective Deboronative Alkynylation under Biomolecule-Compatible Conditions, Huang, Hanchu, Zhang, Guojin, Gong, Li, Zhang, Shuaiyan, Chen, Yiyun, Journal of the American Chemical Society (2014), 136(6), 2280-2283. DOI:10.1021/ja413208y
7.) Stereospecific Cross-Coupling of Secondary Alkyl β-Trifluoroboratoamides, Sandrock, Deidre L., Jean-Gerard, Ludivine, Chen, Cheng-Yi, Dreher, Spencer D., Molander, Gary A., Journal of the American Chemical Society (2010), 132(48), 17108-17110. DOI:10.1021/ja108949w
8.) Aryl Trifluoroborates in Suzuki-Miyaura Coupling: The Roles of Endogenous Aryl Boronic Acid and Fluoride, Butters, Mike, Harvey, Jeremy N., Jover, Jesus, Lennox, Alastair J. J., Lloyd-Jones, Guy C., Murray, Paul M., Angewandte Chemie, International Edition (2010), 49(30), 5156-5160, S5156/1-S5156/68.
9.) Iridium-Catalyzed Enantioselective Allylic Vinylation, Hamilton, James Y., Sarlah, David, Carreira, Erick M., Journal of the American Chemical Society (2013), 135(3), 994-997. DOI:10.1021/ja311422z
10.) Organocatalytic Vinyl and Friedel-Crafts Alkylations with Trifluoroborate Salts, Lee, Sandra, MacMillan, David W. C., Journal of the American Chemical Society (2007), 129(50), 15438-15439. DOI:10.1021/ja0767480