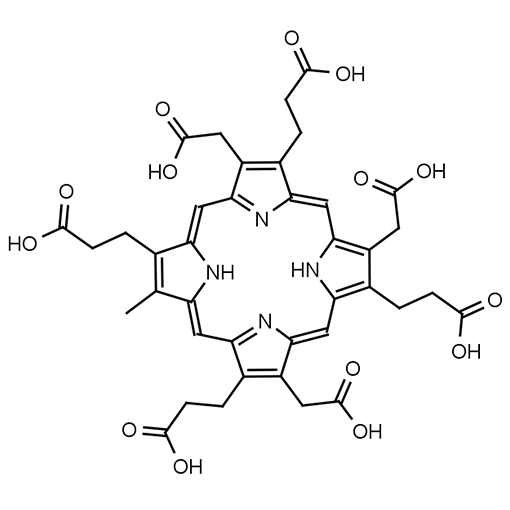
Heptacarboxylporphyrin I
See related porphyria research compounds:
Molecular Formula: C39H38N4O14
CAS#: N/A
SMILES: CC1=C(CCC(O)=O)/C2=C/C3=C(CC(O)=O)C(CCC(O)=O)=C(N3)/C=C4C(CC(O)=O)=C(CCC(O)=O)C(/C=C5N/C(C(CCC(O)=O)=C5CC(O)=O)=CC1=N2)=N/4
MDL#: N/A
Catalog#: HEPTA1
Molecular weight: 786.75 g/mol
Other names:
Fields of Interest: Heptacarboxylporphyrin I is an important analyte used in the diagnosis of porphyria diseases. It is a decarboxylation product of Uroporphyrin I. The presence of Heptacarboxylporphyrin I in biological samples indicates a malfunction of the heme biosynthesis pathway.
Background & Applications: Heptacarboxylporphyrin I is an important analyte used in the determination of porphyria diseases.
Appearance: Dark purple solid
Purity: ≥95% Heptacarboxylporphyrin, ≥90% Heptacarboxylporphyrin I
Storage: −20 °C
Solubility: Soluble in basic aqueous media (pH >9.5) or in highly acidic media (pH <2). Beware that when dissolved in highly acidic aqueous solutions there may be some degradation of the Heptacarboxylporphyrin I to form decarboxylated byproducts.
Literature:
Harada, Y.; Murayama, Y.; Takamatsu, T.; Otsuji, E.; Tanaka, H. 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence Imaging for Tumor Detection: Recent Advances and Challenges. Int. J. Mol. Sci. 2022, 23, 6478. https://doi.org/10.3390/ijms23126478
Kiening, M.; Lange, N. A Recap of Heme Metabolism towards Understanding Protoporphyrin IX Selectivity in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7974. https://doi.org/10.3390/ijms23147974
Di Pierro, E.; De Canio, M.; Mercadante, R.; Savino, M.; Granata, F.; Tavazzi, D.; Nicolli, A.M.; Trevisan, A.; Marchini, S.; Fustinoni, S. Laboratory Diagnosis of Porphyria. Diagnostics 2021, 11, 1343. https://doi.org/10.3390/diagnostics11081343
Phillips, J. D Heme Biosynthesis and the Porphyrias Molecular Genetics and Metabolism 2019, 3, 164-177. https://doi.org/10.1016/j.ymgme.2019.04.008