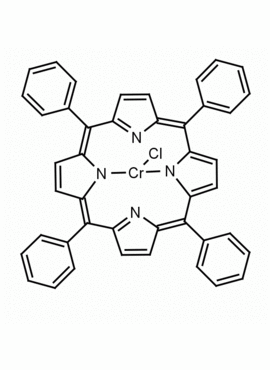
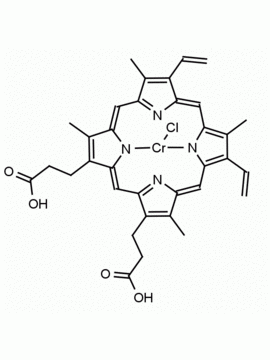
Cr(III) meso-Tetraphenylporphine Chloride (contains 1-3% chlorin) CAS: 28110-70-5 MDL: MFCD0026981
Molecular weight: 700.18 g/mol
Molecular Formula: C44H28ClCrN4
CAS Number: 28110-70-5
Storage: Store at room temperature, protect from light.
Purity: >95%
Synonyms: Salicylate Ionophore I, meso-Tetraphenylporphyrine Chromium(III) chloride. Chromiumtetraphenylporphinechloride, Meso-Tetraphenylporphyrin-Cr(III) chloride, Chromium(III) Tetraphenylporphine Chloride, Chloro(meso- tetraphenylporphinato)chromium, Chromium (III) Tetraphenylporphine Chloride, 5,10,15,20-Tetraphenylporphyrin Cr(III) Chloride, 5,10,15,20-Tetraphenyl-21H,23H- porphine chromium(III), Chromium(III) 5,10,15,20-tetraphenylporphine chloride, 21H,23H-Porphine, 5
Field of Interest: Catalysis, epoxide carbonylation, sensors, ion selective electrodes, Epoxidation
Background: Cr(III) meso-Tetraphenylporphine Chloride (contains 1-3% chlorin) is a synthetically derived metallophorphyrin specialty chemical manufactured by Frontier Specialty Chemicals containing chromium in the (III) oxidation state. This complex has been used for the construction of an salicylate-selective membrane electrode.1 Cr(III) meso-Tetraphenylporphine Chloride in combination with dicobalt octacarbonyl produces a highly active catalytic species for the carbonylation of epoxides to B-lactones, avoiding the need to handle air sensitive intermediates for the preparation of the Coates catalyst.2 Cr(III) meso-Tetraphenylporphine Chloride was used to polymerize styrene oxide catalytically under various conditions in the presence of bis(triphenylphosphine)iminium chloride.3 Exo-selective epoxidation of norbornene was accomplished using Cr(III) meso-Tetraphenylporphine Chloride as a catalyst.4 Cr(III) meso-Tetraphenylporphine Chloride catalyzed the dehydrogenative synthesis of imines from alcohols and amines.5 Cr(III) meso-Tetraphenylporphine Chloride was used to synthesize a selectively functionalized polylactide polymer.6
References:
1.) Shahrokhian, S., Hamzehloei, A., Bagherzadeh, M.; Chromium(III) Porphyrin as a selective ionophore in a salicylate-selective membrane electrode. Anal Chem. 2002,14, 3312-3320. doi: 10.1021/ac020099n.
2.) Ganji, P., Doyle, D.J., Ibrahim, H.; In Situ Generation of the Coates Catalyst: A Practical and Versatile Catalytic System for the Carbonylation of meso-Epoxides. Lett. 2011, 12, 3142-3145. https://doi.org/10.1021/ol201043d
3.) Harrold, et al. Studies of Ring-Opening Reactions of Styrene Oxide by Chromium Tetraphenylporphyrin Initiators. Mechanistic and Stereochemical Considerations. Macromolecules 2013, 46, 3, 692–698. https://doi.org/10.1021/ma302492p
4.) Traylor, et al. Alkene epoxidations catalyzed by iron(III), manganese(III), and chromium(III) porphyrins. Effects of metal and porphyrin substituents on selectivity and regiochemistry of epoxidation. J. Am. Chem. Soc. 1989, 111, 19, 7443–7448. https://doi.org/10.1021/ja00201a026
5.) Miao, et al. Vanadium- and Chromium-Catalyzed Dehydrogenative Synthesis of Imines from Alcohols and Amines. Organometallics 2021, 40, 9, 1328–1335. https://doi.org/10.1021/acs.organomet.1c00123
6.) Balasanthiran, et al. Coupling of Propylene Oxide and Lactide at a Porphyrin Chromium(III) Center. J. Am. Chem. Soc. 2015, 137, 5, 1786–1789. https://doi.org/10.1021/ja512554t