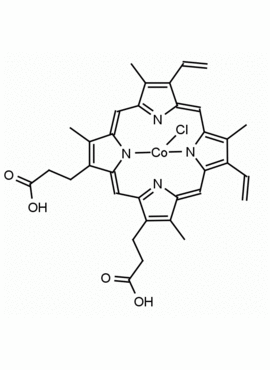
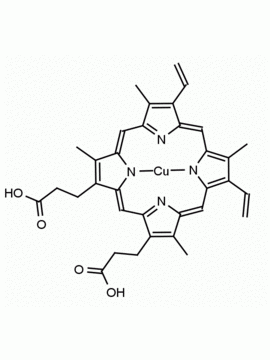
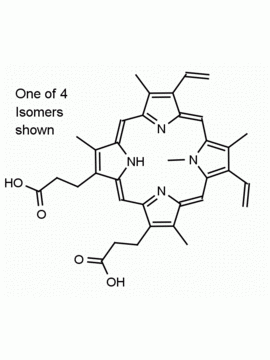
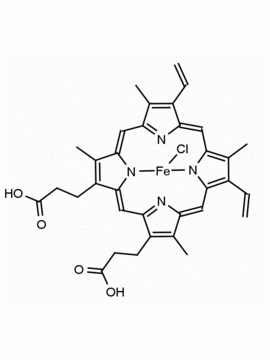
Co(III) Protoporphyrin IX chloride 8,13-Divinyl-3,7,12,17-tetramethyl-21H,23H-porphine-2,18-dipropionic acid cobalt(III) chloride CAS: 102601-60-5 MDL: MFCD00216774
Field of Interest: Natural Products Synthesis, Catalysis, Heme Oxygenase Pathway, Inflammation
Background: Co(III) Protoporphyrin IX chloride is a porphyrin based natural product that can catalyze the production of hydrocarbons and carbon monoxide from an aldehyde, and can act as a sensing device on optical surfaces. 1,2 Medically, the compound up-regulates heme-oxygenase (HO-1) and can modulate the HO-1/carbon monoxide axis in inflammatory injury and myocardial infarction.3,4 Cobalt (III) protoporphyrin, when inserted into myoglobin serves as a catalyst for the reduction of N2O to N2.5 Co(III) and Sn(IV) protoporphyrin derivatives inactivate arboviruses including Chikungunya and Zika by targeting the viral envelope.6
Molecular weight: 655.04 g/mol
Molecular Formula: C34H32ClCoN4O4
CAS Number: 102601-60-5
Storage: Store at room temperature, protected from light
Synonyms: MFCD00216774, Protoporphyrin IX cobalt chloride, 8,13-DIVINYL-3,7,12,17-TETRAMETHYL-21H, 23H- PORPHINE-2, 18-DIPROPIONIC ACID COBALT(III) CHLORIDE
References:
1.) Dennis, Michael; Kolattukudy, P. E., A cobalt-porphyrin enzyme converts a fatty aldehyde to a hydrocarbon and carbon monoxide, Proceedings of the National Academy of Sciences of the United States of America (1992), 89(12), 5306-10. DOI:10.1073/pnas.89.12.5306.
2.) Botelho do Rego, A. M.; Ferraria, A. M.; Rei Vilar, M., Grafting of Cobaltic Protoporphyrin IX on Semiconductors toward Sensing Devices: Vibrational and Electronic High-Resolution Electron Energy Loss Spectroscopy and X-ray Photoelectron Spectroscopy Study, Journal of Physical Chemistry C (2013), 117(43), 22298-22306. DOI:10.1021/jp402032x.
3.) Onyiah, Joseph C.; Sheikh, Shehzad Z.; Maharshak, Nitsan; Steinbach, Erin C.; Russo, Steven M.; Kobayashi, Taku; Mackey, Lantz C.; Hansen, Jonathan J.; Moeser, Adam J.; Rawls, John F.; et al, Carbon Monoxide and Heme Oxygenase-1 Prevent Intestinal Inflammation in Mice by Promoting Bacterial Clearance, Gastroenterology (2013), 144(4), 789-798. .2012.12.025.
4.) Lakkisto, Paivi; Kyto, Ville; Forsten, Hanna; Siren, Juha-Matti; Segersvard, Heli; Voipio-Pulkki, Liisa-Maria; Laine, Mika; Pulkki, Kari; Tikkanen, Ilkka, Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1α, SDF-1α and VEGF-B, European Journal of Pharmacology (2010), 635(1-3), 156-164, DOI:10.1016/j.ejphar.2010.02.050
5.) Rapson, T. D.; Warneke, S.; Musameh, M. M.; Dacres, H.; Macdonald, Ben, C.T.; Trowell, Stephen, C., Conversion of Nitrous Oxide to Nitrogen by Cobalt-Substituted Myoglobin. RSC Adv., 2-15, 5- 89003- 89008.
6.) Neris, Romulo, L.S., et. al. Co-Protoporphyrin IX and Sn-protoporphyrin IX inactivate Zika, Chikungunya and other arboviruses by targeting the viral envelope. Scientific Reports., 8, 2018.