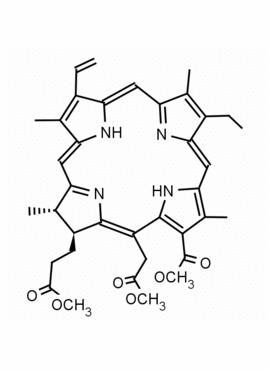
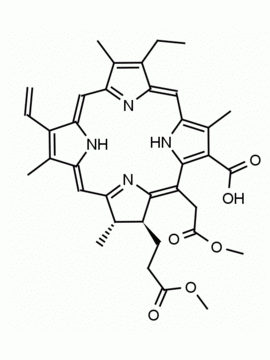
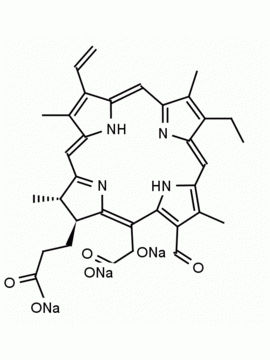
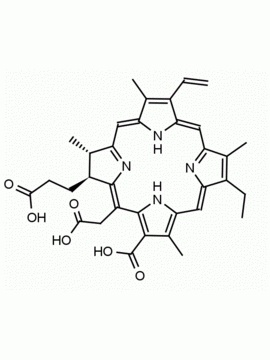
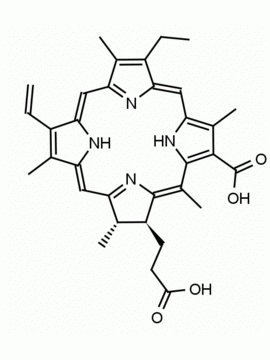
Chlorin e6 trimethyl ester CAS: 35038-32-5 MDL:MFCD09838560
Field of Interest: Natural Products Synthesis, Photodynamic Therapy, Photophysics
Background: Chlorin e6 trimethyl ester Is active as a photosensitizing agent in photodynamic therapy and a well studied compound by R. B. Woodward, elaborating synthesis of chlorins and compounds related to chlorophyll 1,2 Metallation and reactions of the ring system are extensively studied,3 and the mono L- aspartylchlorin-e6 analog is known as Talaporfin, an important clinical agent.4
Molecular weight: 638.8 g/mol
Molecular Formula: C37H42N4O6
CAS Number: 35038-32-5
Storage: Store at room temperature
Synonyms: Chlorin-e6-trimethyl ester, Methyl (2S-trans)-13-ethyl-2,3-dihydro-18-(methoxycarbonyl)-20-(2-methoxy-2-oxoethyl)-3,7,12,17-tetramethyl-8-vinyl-21H,23H-porphine-2-propionate, Chlorine e6, trimethyl ester, EINECS 252-329-6, NSC75785, NSC-75785
References:
1.) Pandey, Ravindra K.; Bellnier, David A.; Smith, Kevin M.; Dougherty, Thomas J., Chlorin and porphyrin derivatives as potential photosensitizers in photodynamic therapy. Photochemistry and Photobiology (1991), 53(1), 65-72.
2.) Woodward, R. B.; Skaric, Vinko, A new aspect of the chemistry of chlorins, Journal of the American Chemical Society (1961), 83, 4676-8. And Woodward, R. B.; Ayer, W. A.; Beaton, J. M.; Bickelhaupt, F.; Bonnett, R.; Buchschacher, P.; Closs, G. L.; Dutler, H.; Hannah, J.; Hauck, F. P.; et al. The total synthesis of chlorophyll, Journal of the American Chemical Society (1960), 82, 3800-2. DOI:10.1021/ja01499a093.
3.) O’Shea, Donal F.; Miller, Mark A.; Matsueda, Hiroko; Lindsey, Jonathan S., Investigation of the Scope of Heterogeneous and Homogeneous Procedures for Preparing Magnesium Chelates of Porphyrins, Hydroporphyrins, and Phthalocyanines, Inorganic Chemistry (1996), 35(25), 7325-7338. DOI:10.1021/IC960812P.
4.) Hargus, Jodie A.; Fronczek, Frank R.; Vicente, M. Graca H.; Smith, Kevin M., Mono-(L)-aspartylchlorin-e6, Photochemistry and Photobiology (2007), 83(5), 1006-1015. DOI:10.1111/j.1751-1097.2007.00092.x
Alternative Names
(17S,18S)-7-Éthyl-20-(2-méthoxy-2-oxoéthyl)-18-(3-méthoxy-3-oxopropyl)-3,8,13,17-tétraméthyl-12-vinyl-17,18-dihydro-2-porphyrinecarboxylate de méthyle
21H,23H-Porphine-2-propanoic acid, 8-ethenyl-13-ethyl-2,3-dihydro-18-(methoxycarbonyl)-20-(2-methoxy-2-oxoethyl)-3,7,12,17-tetramethyl-, methyl ester, (2S,3S)-
Methyl (17S,18S)-7-ethyl-20-(2-methoxy-2-oxoethyl)-18-(3-methoxy-3-oxopropyl)-3,8,13,17-tetramethyl-12-vinyl-17,18-dihydro-2-porphyrincarboxylate
methyl (2S-trans)-13-ethyl-2,3-dihydro-18-(methoxycarbonyl)-20-(2-methoxy-2-oxoethyl)-3,7,12,17-tetramethyl-8-vinyl-21H,23H-porphine-2-propionate
Methyl-(17S,18S)-7-ethyl-20-(2-methoxy-2-oxoethyl)-18-(3-methoxy-3-oxopropyl)-3,8,13,17-tetramethyl-12-vinyl-17,18-dihydro-2-porphyrincarboxylat
Chlorin-e6-trimethyl ester
METHYL-(2S-TRANS)-13-ETHYL-2,3-DIHYDRO-18-(METHOXYCARBONYL)-20-(2-METHOXY-2-OXOETHYL)-3,7,12,17-TETRAMETHYL-8-VINYL-21H,23H-PORPHINE-2-PROPIONATE