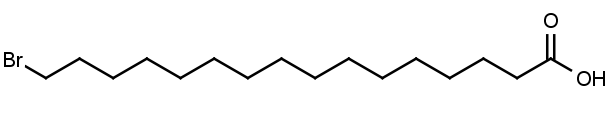
16-Bromohexadecanoic acid
Molecular Formula: C16H31BrO2
CAS#: 2536-35-8
MDL: MFCD00040779
Catalog #: B36068
Molecular weight: 335.32 g/mol
Other names:
- 16-Bromo-Hexadecanecarboxylic Acid
- 16-bromohexadecanec
arboxylicacid - 16-bromo-hexadecano
ic acid
Fields of Interest: Organic Synthesis, Fatty acids, Pharmaceutical Research, Carboxylic Acids, Building Blocks, Halides, Bifunctional Compounds, Supramolecular Chemistry
Background & Applications: 16-Bromohexadecanoic acid was used in the synthesis of disulfide-tethered diacylglycerols.1 16-Bromohexadecanoic acid was used to create a porphyrin containing a long chain which was subsequently functionalized by thiol displacement, this adduct was used to functionalize gold nanoparticles.2 16-Bromohexadecanoic acid was used to functionalize a naphthoquinone derivative which was subsequently used as a molecular wire attached to a gold surface to investigate energy transfer.3 16-Bromohexadecanoic acid was used in the synthesis of a proteolysis targeting chimera (PROTAC) degrader of NSD3 histone methyltransferases.4
Purity: >95%
Storage: Store at room temperature under inert atmosphere in a dark place
References:
1.) Bhattacharya, et al. Synthesis of Macrocyclic Diacyl/Dialkyl Glycerols Containing Disulfide Tether and Studies of Their Effects upon Incorporation in DPPC Membranes. Implications in the Design of Phospholipase A2 Modulators. J. Org. Chem. 1998, 63, 25, 9232–9242. https://doi.org/10.1021/jo980866b
2.) Hasobe, et al. Photovoltaic Cells Using Composite Nanoclusters of Porphyrins and Fullerenes with Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 4, 1216–1228. https://doi.org/10.1021/ja047768u
3.) Plugging a Molecular Wire into Photosystem I: Reconstitution of the Photoelectric Conversion System on a Gold Electrode. Angewandte Chemie – International Edition, 2009, vol. 48, # 9, p. 1585 – 1587. https://onlinelibrary.wiley.com/doi/10.1002/anie.200805748
4.) Sun, et al. Discovery of a potent and selective proteolysis targeting chimera (PROTAC) degrader of NSD3 histone methyltransferase. European Journal of Medicinal Chemistry, 2022, vol. 239, art. no. 114528. https://doi.org/10.1016/j.ejmech.2022.114528