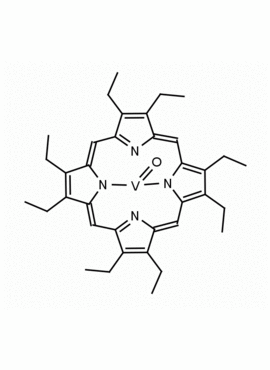
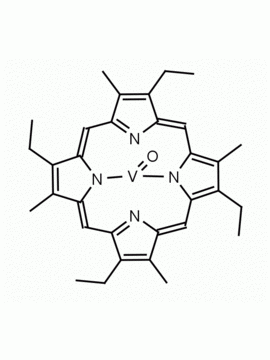
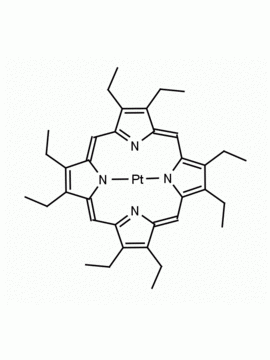
Vanadyl octaethylporphine 2,3,7,8,12,13,17,18-Octaethyl-21H,23H-porphine vanadium (IV) oxide CAS: 27860-55-5 MDL: MFCD00058868
Sizes Available: 100 mg, 25o mg, 1 g, 5 g and larger sizes available
Molecular weight: 599.702 g/mol
Molecular Formula: C36H44N4OV
CAS Number: 27860-55-5
Storage: Store at room temperature, protect from light
Synonyms: [2,3,7,8,12,13,17,18-Octaethylporphyrinato(2-)-κ2N21,N23](oxo)vanadium,[2,3,7,8,12,13,17,1
Field of Interest: Models for Geoporphyrins, Photophysics
Background: Vanadyl octaethylporphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. Vanadyl octaethylporphine is often used as a model for Vanadyl Petroporphyrins.
References:
1.) Kandrashkin, et al. Light-Induced Electron Spin Polarization in Vanadyl Octaethylporphyrin: I. Characterization of the Excited Quartet State. J. Phys. Chem. A 2006, 110, 31, 9607–9616. https://doi.org/10.1021/jp0620365
2.) Kandrashkin, et al. Light-Induced Electron Spin Polarization in Vanadyl Octaethylporphyrin: II. Dynamics of the Excited States. J. Phys. Chem. A 2006, 110, 31, 9617–9626. https://doi.org/10.1021/jp062037x
3.) Bonnett, et al. Chemistry of Vanadyl Porphyrins. Tetrahedron. Volume 34, Issue 3, 1978, Pages 379-385. https://doi.org/10.1016/S0040-4020(01)93596-3
4.) Miyake, et al. Scanning tunneling microscopy investigation of vanadyl and cobalt(II) octaethylporphyrin self-assembled monolayer arrays on graphite. Colloids and Surfaces A: Physicochemical and Engineering Aspects. Volumes 313–314, 1 February 2008, Pages 230-233. https://doi.org/10.1016/j.colsurfa.2007.05.073
5.) Chen, et al. Metal Porphyrin Adsorption onto Asphaltene in Pentane Solution: A Comparison between Vanadyl and Nickel Etioporphyrins. Energy Fuels 2017, 31, 4, 3592–3601.https://doi.org/10.1021/acs.energyfuels.6b03100
6.) Ghosh, et al. Axial Ligand Coordination in Sterically Strained Vanadyl Porphyrins: Synthesis, Structure, and Properties. Inorg. Chem. 2008, 47, 21, 9848–9856. https://doi.org/10.1021/ic800714w
7.) Mironov, N.A., Milordov, D.V., Abilova, G.R. et al. Methods for Studying Petroleum Porphyrins (Review). Pet. Chem. 59, 1077–1091 (2019). https://doi.org/10.1134/S0965544119100074
8.) Mandal, et al. Non-catalytic vanadium removal from vanadyl etioporphyrin (VO-EP) using a mixed solvent of supercritical water and toluene: A kinetic study. Fuel. Volume 92, Issue 1, February 2012, Pages 288-294. https://www.sciencedirect.com/science/article/abs/pii/S0016236111003991