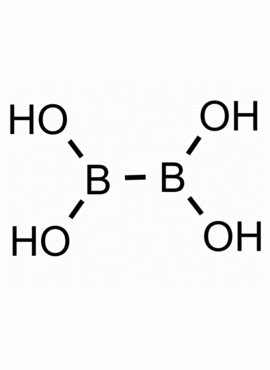
Tetrahydroxydiboron CAS: 13675-18-8 MDL: MFCD05663888
Molecular weight: 89.65 g/mol
Molecular Formula: H4B2O4
CAS Number: 13675-18-8
Storage: Store at 2-8 Co, under dry conditions.
Synonyms: Tetrahydroxydiboron, hypodiboric acid, 13675-18-8, tetrahydroxydiborane, Hypoboric Acid, (dihydroxyboranyl)boronic acid, Diboron tetrahydroxide
Uses: Synthesis of boronic acids, Reducing agent, Organic Synthesis Reagent
Tetrahydroxydiboron is a synthetic specialty chemical useful in the synthesis of pharmaceuticals and specialty organic chemicals.
Selected Uses and Synthesis References:
1.) Cui, Qiang, Djamaladdin G. Musaev, and Keiji. Morokuma. Why Do Pt(PR3)2 Complexes Catalyze the Alkyne Diboration Reaction, but Their Palladium Analogs Do Not? A Density Functional Study. Organometallics 17, no. (1998): 742–51. https://doi.org/10.1021/OM970277G.
2.) Feng, Zhang, Qiao-Qiao Min, Xia-Ping Fu, Lun An, and Xingang. Zhang. Chlorodifluoromethane-Triggered Formation of Difluoromethylated Arenes Catalysed by Palladium. Nature Chemistry 9, no. (2017): 918–23. https://doi.org/10.1038/nchem.2746.
3.) Hu, Dawei, Linghua Wang, and Pengfei. Li. Decarboxylative Borylation of Aliphatic Esters under Visible-Light Photoredox Conditions. Organic Letters 19, no. (2017): 2770–73. https://doi.org/10.1021/acs.orglett.7b01181.
4.) Medina, Dana D., Veronika Werner, Florian Auras, Raphael Tautz, Mirjam Dogru, Joerg Schuster, Stephanie Linke, et al. Oriented Thin Films of a Benzodithiophene Covalent Organic Framework. ACS Nano 8, no. (2014): 4042–52. https://doi.org/10.1021/nn5000223.
5.) Mfuh, Adelphe M., John D. Doyle, Bhuwan Chhetri, Hadi D. Arman, and Oleg V. Larionov. Scalable, Metal- and Additive-Free, Photoinduced Borylation of Haloarenes and Quaternary Arylammonium Salts. Journal of the American Chemical Society 138, no. (2016): 2985–88. https://doi.org/10.1021/jacs.6b01376.
6.) Molander, Gary A., Sarah L. J. Trice, and Spencer D. Dreher. Palladium-Catalyzed, Direct Boronic Acid Synthesis from Aryl Chlorides: A Simplified Route to Diverse Boronate Ester Derivatives. Journal of the American Chemical Society 132, no. (2010): 17701–3. https://doi.org/10.1021/ja1089759.
7.) Molander, Gary A., Sarah L. J. Trice, Steven M. Kennedy, Spencer D. Dreher, and Matthew T. Tudge. Scope of the Palladium-Catalyzed Aryl Borylation Utilizing Bis-Boronic Acid. Journal of the American Chemical Society 134, no. (2012): 11667–73. https://doi.org/10.1021/ja303181m.
8.) Olsson, Vilhelm J., Sara Sebelius, Nicklas Selander, and Kalman J. Szabo. Direct Boronation of Allyl Alcohols with Diboronic Acid Using Palladium Pincer-Complex Catalysis. A Remarkably Facile Allylic Displacement of the Hydroxy Group under Mild Reaction Conditions. Journal of the American Chemical Society 128, no. (2006): 4588–89. https://doi.org/10.1021/ja060468n.
9.) Sebelius, Sara, Vilhelm J. Olsson, and Kalman J. Szabo. Palladium Pincer Complex Catalyzed Substitution of Vinyl Cyclopropanes, Vinyl Aziridines, and Allyl Acetates with Tetrahydroxydiboron. An Efficient Route to Functionalized Allylboronic Acids and Potassium Trifluoro(Allyl)Borates. Journal of the American Chemical Society 127, no. (2005): 10478–79. https://doi.org/10.1021/ja052885q.
10.) Selander, Nicklas, Andreas Kipke, Sara Sebelius, and Kalman J. Szabo. Petasis Borono-Mannich Reaction and Allylation of Carbonyl Compounds via Transient Allyl Boronates Generated by Palladium-Catalyzed Substitution of Allyl Alcohols. An Efficient One-Pot Route to Stereodefined α-Amino Acids and Homoallyl Alcohols. Journal of the American Chemical Society 129, no. (2007): 13723–31. https://doi.org/10.1021/ja074917a.