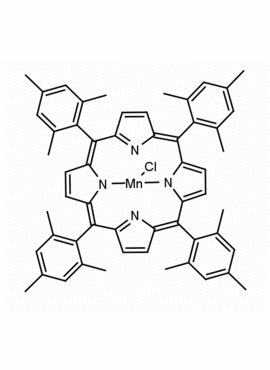
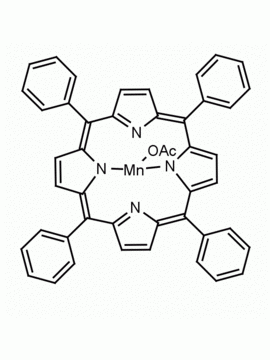
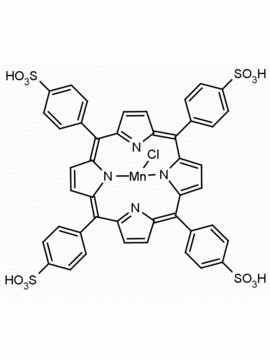
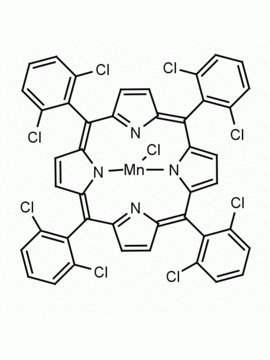
Mn(III) meso-Tetra(2,4,6-trimethylphenyl)porphine chloride, CAS: 85939-49-7 MDL: MFCD22377371
Molecular weight: 871.430 g/mol
Molecular Formula: C56H52ClMnN
CAS Number: 85939-49-7
Storage: Store at room temperature, protect from light
Synonyms: Chlor[5,10,15,20-te
Field of Interest: Catalysis, fluoro-decarboxylation, alkane hydroxylation, epoxidation, Cytochrome P450 mimics
Background: Mn(III) meso-Tetra(2,4,6-trimethylphenyl)porphine chloride is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. Mn(III) meso-Tetra(2,4,6-trimethylphenyl)porphine chloride mediated 18F-fluorodecarboxylation of flour phenyl acetic acid derivatives to afford 18F-difluoromethylarenes.1 Kinetic isotope studies have been conducted on the catalytic alkane dihydroxylations mediated by Mn(III) meso-Tetra(2,4,6-trimethylphenyl)porphine chloride.2 The reaction of hydroxide ion with Mn(III) meso-Tetra(2,4,6-trimethylphenyl)porphine chloride in coordinating solids leads to the reduction of Mn(III) to Mn(II), chemistry which is relevant to the chemistry of photosystem II.3 meso-Tetra(2,4,6-trimethylphenyl)porphine chloride catalytically epoxidizes alkenes in the presence of hypochlorite ions.4
References:
1.) Sap, et al. Synthesis of 18F-difluoromethylarenes using aryl boronic acids, ethyl bromofluoroacetate and [18F]fluoride. Chem. Sci., 2019, 10, 3237-3241. 10.1039/C8SC05096A
2.) Sorokin, et al. Intramolecular kinetic isotope effects in alkane hydroxylations catalyzed by manganese and iron porphyrin complexes. J. Am. Chem. Soc. 1993, 115, 16, 7293–7299. https://doi.org/10.1021/ja00069a031
3.) Nakagaki, et al. Relevance of the reaction of a manganese(III) chelate with hydroxide ion to photosynthesis: Reaction of hydroxide ion with 5,10,15,20-tetrakis(2,4,6-trimethylphenyl)porphinatomanganese(III) in ligating and non ligating solvents. Proc. Natl. Acad. Sci. USA Vol. 85, pp. 5424-5428, August 1988.
4.) van der Made, et al. Effect of Ph on the Epoxidation of Alkenes by the Cytochrome-P-450 Model Manganese(Iii) Porphyrin Hypochlorite. Recueil des Travaux Chimiques des Pays-Bas. Volume 108, Issue 5, 1989,Pages 185-188. https://doi.org/10.1002/recl.19891080505