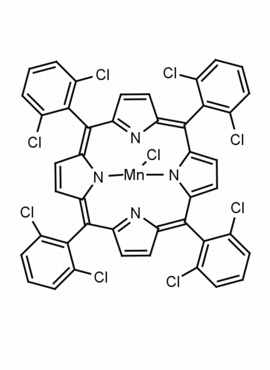
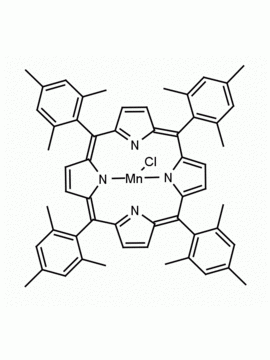
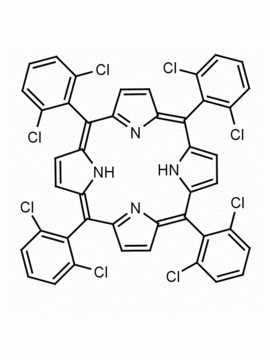
Mn(III) meso-Tetra (o-dichlorophenyl) porphine chloride CAS: 91463-17-1 MDL: MFCD06227756
Molecular weight: 993.71 g/mol
Molecular Formula: C45H23Cl9MnN4
CAS Number: 91463-17-1
Storage: Store at room temperature, protect from light.
Synonyms: 21H,23H-Porphine, 5
Fields of Interest: Synthetic Porphyrins, Catalysis, Cytochrome P450 mimics
Background: Mn(III) meso-Tetra (o-dichlorophenyl) porphine chloride is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Battioni, et al. Monooxygenase-like Oxidation of Hydrocarbons by H2O2 Catalyzed by Manganese Pophyrins and Imidazole: Selection of the Best Catalytic System and Nature of the Active Oxygen Species. Journal of the American Chemical Society, 1988, vol. 110, # 25, p. 8462 – 8470. https://doi.org/10.1021/ja00233a023
2.) Mansuy, et al. Highly oxidation resistant inorganic-porphyrin analogue polyoxometalate oxidation catalysts. 2. Catalysis of olefin epoxidation and aliphatic and aromatic hydroxylations starting from α2-P2W17O61(M n+·Br)(n-11) (Mn+ = Mn3+, Fe3+, Co2+, Ni2+, Cu2+). Journal of the American Chemical Society, 1991, vol. 113, # 19, p. 7222 – 7226. https://doi.org/10.1021/ja00019a019
3.) Chan, et al. Oxidative amide synthesis and N-terminal α-amino group ligation of peptides in aqueous medium. Journal of the American Chemical Society, 2006, vol. 128, # 46, p. 14796 – 14797. https://doi.org/10.1021/ja064479s
4.) Guo, et al. Highly Reactive Manganese(IV)-Oxo Porphyrins Showing Temperature-Dependent Reversed Electronic Effect in C-H Bond Activation Reactions. Journal of the American Chemical Society, 2019, vol. 141, # 31, p. 12187 – 12191. https://doi.org/10.1021/jacs.9b04496
5.) Akagah, et al. Oxidation of antiparasitic 2-substituted quinolines using metalloporphyrin catalysts: Scale-up of a biomimetic reaction for metabolite production of drug candidates. Organic and Biomolecular Chemistry, 2008, vol. 6, # 24, p. 4494 – 4497. https://pubs.rsc.org/en/content/articlelanding/2008/OB/b815963g
6.) Zhang, et al. Unprecedented Reactivities of Highly Reactive Manganese(III)-Iodosylarene Porphyrins in Oxidation Reactions. Journal of the American Chemical Society, 2020, vol. 142, # 47, p. 19879 – 19884. https://doi.org/10.1021/jacs.0c10159
7.) Che, et al. Direct preparation of unprotected aminimides (R3N+-NH–) from natural aliphatic tertiary alkaloids (R3N) by [Mn(TDCPP)Cl]-catalysed: N -amination reaction. Chemical Communications, 2020, vol. 56, # 64, p. 9102 – 9105. https://doi.org/10.1039/D0CC02934C
8.) Bhuvanesh, et al. Nitrene Photochemistry of Manganese N-Haloamides. Angewandte Chemie – International Edition, 2021, vol. 60, # 51, p. 26647 – 26655. https://doi.org/10.1002/anie.202108304