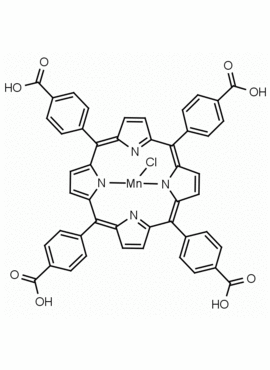
Mn(III) meso-Tetra (4-carboxyphenyl) porphine chloride CAS: 55266-18-7 MDL: MFCD06227760
Molecular weight: 879.2 g/mol
Molecular Formula: C48H28MnNO8 Cl
CAS Number: 55266-18-7
Storage: Store at room temperature, protect from light
Synonyms: mntbap, Bio2_000348, Mn(III) Meso-Tetra (4-carboxyphenyl) porphine chloride, Manganese(III) meso-tetra(4-carboxyphenyl)porphine chloride, Chlor[4,4′,4”,4”’
Fields of Interest: Metal Organic Frameworks, Covalent Organic Frameworks, Nanoparticles, Catalysis, Surface-initiated atom transfer radical polymerization (SI-ATRP)
Background: Mn(III) meso-Tetra (4-carboxyphenyl) porphine chloride is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. Mn(III) meso-Tetra (4-carboxyphenyl) porphine chloride was used to initiate surface-initiated atom transfer radical polymerization (SI-ATRP) for the construction of a thrombin biosensor.1 Mn(III) meso-Tetra (4-carboxyphenyl) porphine chloride was used to study the self-assembly behavior of porphyrins in crystals.2 Multifunctional photocatalysis where produced from metallated meso-tetra(4-carboxyphenyl)porphine derivatives including Mn(III) meso-Tetra (4-carboxyphenyl) porphine chloride exhibiting catalytic photo degradation and bactericidal properties.3 A MOF was constructed using Mn(III) meso-Tetra (4-carboxyphenyl) porphine chloride and found to be catalytically active towards the epoxidation of alkenes with molecular oxygen.4
T40904 Specific References:
1.) Liu, et al. Highly Sensitive Thrombin Detection by Combination of Click Chemistry and Surface-Initiated Polymerization. J. Electrochem. Soc. 166 B1387.
2.) Shmilovits, et al. Self-assembly patterns of porphyrins in crystals. Structures of hydrogen-bonding and coordination polymers of manganese tetra(carboxyphenyl)porphyrin. New J. Chem., 2004,28, 223-227.
3.) Chanhom, et. al. Metalloporphyrins-sensitized titania-silica-iron oxide nanocomposites with high photocatalytic and bactericidal activities under visible light irradiation. Journal of Magnetism and Magnetic Materials Volume 475, 1 April 2019, Pages 602-610.
4.) Brown, et al. Epoxidation of alkenes with molecular oxygen catalyzed by a manganese porphyrin-based metal–organic framework. Catalysis Communications Volume 59, 10 January 2015, Pages 50-54
Selected Metal-Organic Framework And Nano Assemblies Literature
1.) Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal-Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts, Feng, Dawei, Gu, Zhi-Yuan, Li, Jian-Rong, Jiang, Hai-Long, Wei, Zhangwen, Zhou, Hong-Cai, Angewandte Chemie, International Edition (2012), 51(41), 10307-10310, S10307/1-S10307/46. DOI:10.1002/anie.201204475
2.) Highly Stable Zr(IV)-Based Metal-Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water, Wang, Bin, Lv, Xiu-Liang, Feng, Dawei, Xie, Lin-Hua, Zhang, Jian, Li, Ming, Xie, Yabo, Li, Jian-Rong, Zhou, Hong-Cai, Journal of the American Chemical Society (2016), 138(19), 6204-6216. DOI:10.1021/jacs.6b01663
3.) Memory of macromolecular helicity assisted by interaction with achiral small molecules, Yashima, Eiji, Maeda, Katsuhiro, Okamoto, Yoshio, Nature (London) (1999), 399(6735), 449-451. DOI:10.1038/20900
4.) Construction of Ultrastable Porphyrin Zr Metal-Organic Frameworks through Linker Elimination, Feng, Dawei, Chung, Wan-Chun, Wei, Zhangwen, Gu, Zhi-Yuan, Jiang, Hai-Long, Chen, Ying-Pin, Darensbourg, Donald J., Zhou, Hong-Cai,Journal of the American Chemical Society (2013), 135(45), 17105-17110. DOI:10.1021/ja408084
5.) Design, Synthesis, Structure, and Gas (N2, Ar, CO2, CH4, and H2) Sorption Properties of Porous Metal-Organic Tetrahedral and Heterocuboidal Polyhedra, Sudik, Andrea C., Millward, Andrew R., Ockwig, Nathan W., Cote, Adrien P., Kim, Jaheon, Yaghi, Omar M., Journal of the American Chemical Society (2005), 127(19), 7110-7118. DOI:10.1021/ja042802q
6.) Synthesis, Structure, and Metalation of Two New Highly Porous Zirconium Metal-Organic Frameworks, Morris, William, Volosskiy, Boris, Demir, Selcuk, Gandara, Felipe, McGrier, Psaras L., Furukawa, Hiroyasu, Cascio, Duilio, Stoddart, J. Fraser, Yaghi, Omar M., Inorganic Chemistry (2012), 51(12), 6443-6445. DOI:10.1021/ic300825s
7.) A Catalytically Active, Permanently Microporous MOF with Metalloporphyrin Struts, Shultz, Abraham M., Farha, Omar K., Hupp, Joseph T., Nguyen, SonBinh T., Journal of the American Chemical Society (2009), 131(12), 4204-4205. DI:10.1021/ja900203f
8.) Dye-Sensitized Nanostructured p-Type Nickel Oxide Film as a Photocathode for a Solar Cell, He, Jianjun, Lindstroem, Henrik, Hagfeldt, Anders, Lindquist, Sten-Eric, Journal of Physical Chemistry B (1999), 103(42), 8940-8943. DOI:10.1021/jp991681r