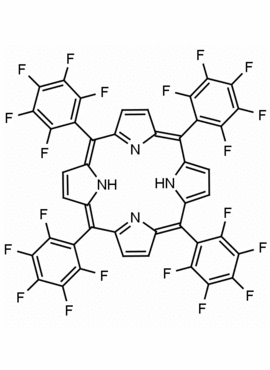
meso-Tetra(pentafluorophenyl)porphine chlorin free 5,10,15,20-Tetrakis(pentafluorophenyl)-21H,23H-porphine CAS: 25440-14-6 MDL: MFCD00010032
Molecular weight: 974.55 g/mol
Molecular Formula: C44H10F20N4
CAS Number: 25440-14-6
Storage: Store at room temperature, protected from light
Synonyms: 5,10,15,20-tetrakis pentafluorophenyl porphyrin,5,10,15,20-tetrakispentafluorophenyl-21h,23h-porphine,meso-tetra pentafluorophenyl porphine,21h,23h-porphine, 5,10,15,20-tetrakis pentafluorophenyl,5,10,15,20-tetrakis 2,3,4,5,6-pentafluorophenyl-21,22-dihydroporphyrin
Field of Interest: Combinatorial Synthesis, Photodynamic Therapy, Electrocatalysis, Photo Redox Catalysis
Background: meso-Tetra(pentafluorophenyl)porphine is synthetic porphyrin specialty chemical with reactive fluorine at the para position generating products with nucleophiles. 1,2 Meso-Tetra(pentafluorophenyl)porphine is active as an electrocatalyst for the generation of hydrogen gas in the presence of p-toluenesulfonic acid.3 Meso-Tetra(pentafluorophenyl)porphine is active as a photoredox catalyst for Csp2-H arylation chemistry.4 The ultra-fast photophysics of meso-tetra(pentafluorophenyl)porphine have been studied.5 Thioaryl porphyrins derived from meso-tetra(pentafluorophenyl)porphine where bound to bind DNA.6 [3+2] cycloaddition of iminonitriles to meso-tetra(pentafluorophenyl)porphine gives Pyrazoline-Fused Chlorins which are of interest as PDT photosensitizes.7
References:
1.) Samaroo, D., Vinodu, M., Chen, X., Drain, C. M., meso-Tetra(pentafluorophenyl)porphyrin as an Efficient Platform for Combinatorial Synthesis and the Selection of New Photodynamic Therapeutics using a Cancer Cell Line, Comb. Chem.2007, 9, 06, 998–1011.
2.) Samaroo, D., Soll, E. C., Todar, L.J., Drain, C. M., Efficient Microwave-Assisted Synthesis of Amine-Substituted Tetrakis(pentafluorophenyl)porphyrin, Org. Lett. 2006, 8, 22, 4985–4988.
3.) Wu, et al. Hydrogen gas generation using a metal-free fluorinated porphyrin Chem. Sci., 2018, 9, 4689-4695 DOI: 10.1039/C8SC00093J
4.) de Souza, et al. Porphyrins as Photoredox Catalysts in Csp2–H Arylations: Batch and Continuous Flow Approaches. J. Org. Chem. 2018, 83, 24, 15077–15086.
5.) Hemant Kumar, et al. Ultrafast Relaxation Dynamics of 5,10,15,20-meso-Tetrakis Pentafluorophenyl Porphyrin Studied by Fluorescence Up-Conversion and Transient Absorption Spectroscopy. J. Phys. Chem. A 2015, 119, 8, 1267–1278.
6.) Foletto, et al. A New Protocol for the Synthesis of New Thioaryl-Porphyrins Derived from 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin: Photophysical Evaluation and DNA-Binding Interactive Studies. Molecules 2018, 23(10), 2588; doi.org/10.3390/molecules23102588
7.) Wyrebek, et al. An Approach to Pyrazoline-Fused Chlorins by Dipolar [3 + 2]-Cycloaddition of Iminonitriles to meso-Tetrakis(pentafluorophenyl)porphyrin: Synthesis of New PDT Photosensitizers. Bulletin of the Chemical Society of Japan 2012, Vol.85, No.10 1167-1174https://doi.org/10.1246/bcsj.20110408