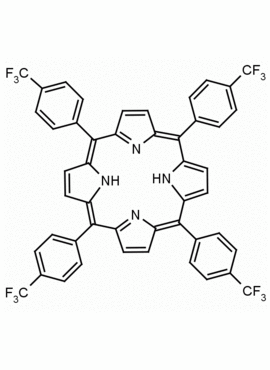
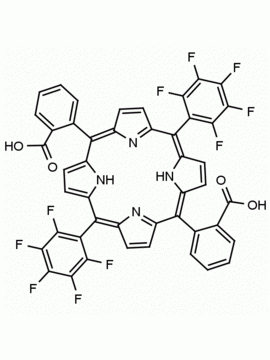
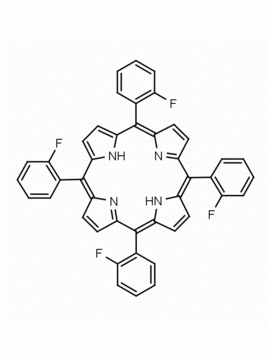
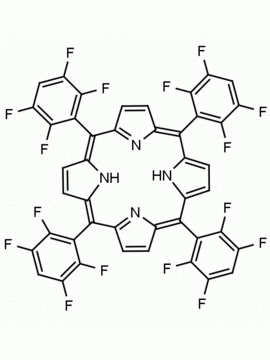
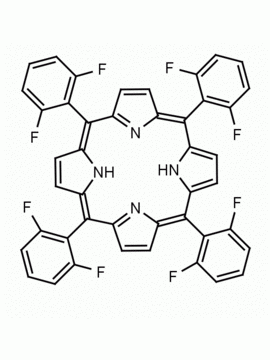
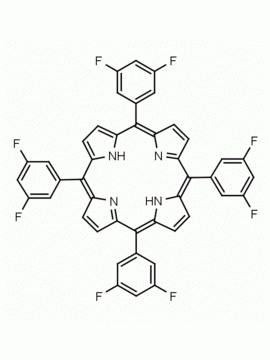
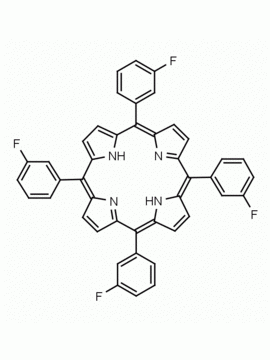
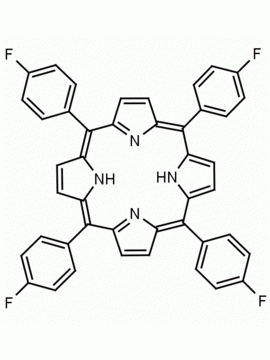
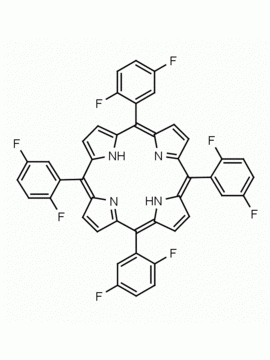
meso-Tetra(4-trifluoromethylphenyl) porphine CAS: 56420-24-7 MDL: MFCD28118891
Molecular weight: 886.73 g/mol
Molecular Formula: C48H26F12N4
CAS Number: 56420-24-7
Storage: Store at room temperature, protect from light.
Synonyms: 21H,23H-Porphine, 5
Fields of Interest: Synthetic Porphyrins, Fluorinated Porphyrins
Background: meso-Tetra(4-trifluoromethylphenyl) porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Jin, et al. Fluoroalkylation of porphyrins: Synthesis and reactions of β-fluoroalkyltetraarylporphyrins. Journal of Organic Chemistry, 2003, vol. 68, # 10, p. 3912 – 3917. https://doi.org/10.1021/jo0207269
2.) Remello, et al. Synthesis of water-soluble silicon-porphyrin: Protolytic behaviour of axially coordinated hydroxy groups. Dalton Transactions, 2015, vol. 44, # 46, p. 20011 – 20020. https://doi.org/10.1039/C5DT03654B
3.) Arunkumar, et al. Structural, spectroscopic and electrochemical investigations on fluorinated meso -tetraaryl porphyrins. Journal of Porphyrins and Phthalocyanines, 2017, vol. 21, # 9, p. 622 – 631. https://doi.org/10.1142/S108842461750064X
4.) Bruckner, et al. Stepwise Preparation of meso-Tetraphenyl- and meso-Tetrakis(4-trifluoromethylphenyl)bacteriodilactones and their Zinc(II) and Palladium(II) Complexes. European Journal of Organic Chemistry, 2020, vol. 2020, # 4, p. 475 – 482. https://doi.org/10.1002/ejoc.201901727
5.) Guergueb, et al. CO2 to CO Electroreduction, Electrocatalytic H2 Evolution, and Catalytic Degradation of Organic Dyes Using a Co(II) meso-Tetraarylporphyrin. Molecules, 2022, vol. 27, # 5, art. no. 1705. https://doi.org/10.3390/molecules27051705
6.) Sugimura, et al. 20π Antiaromatic Isophlorins without Metallation or Core Modification. European Journal of Organic Chemistry, 2022, vol. 2022, # 46, art. no. E202200747. https://doi.org/10.1002/ejoc.202200747