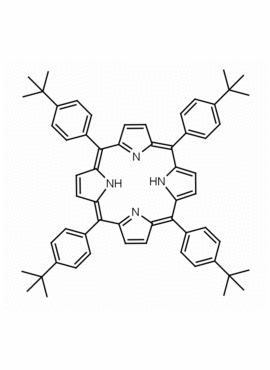
meso-Tetra(4-tert-butylphenyl) Porphine CAS: 110452-48-7 MDL: MFCD20502349
Molecular weight: 839.16 g/mol
Molecular Formula: C60H62N4
CAS Number: 110452-48-7
Storage: Store at room temperature, protect from light.
Synonyms: 21H,23H-Porphine, 5
Fields of Interest: Synthetic Porphyrins
Background: meso-Tetra(4-tert-butylphenyl) Porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Richeter, et al. Synthesis and structural characterisation of the first N-heterocyclic carbene ligand fused to a porphyrin. Chemical Communications, 2007, # 21, p. 2148 – 2150. https://doi.org/10.1039/B704681B
2.) Samankumara, et al. Syntheses, structures, modification, and optical properties of meso-tetraaryl-2,3-dimethoxychlorin, and two isomeric meso-tetraaryl-2,3,12,13- tetrahydroxybacteriochlorins. Organic and Biomolecular Chemistry, 2010, vol. 8, # 8, p. 1951 – 1965. https://doi.org/10.1039/B924539A
3.) Lefebvre, et al. Synthesis, characterization, and electronic properties of metalloporphyrins annulated to exocyclic imidazole and imidazolium rings. European Journal of Organic Chemistry, 2010, # 10, p. 1912 – 1920. https://doi.org/10.1002/ejoc.200901310
4.) Shi, et. al. Porphyrin-containing D-π-A conjugated polymer with absorption over the entire spectrum of visible light and its applications in solar cells. Journal of Materials Chemistry, 2012, vol. 22, # 22, p. 11006 – 11008. https://doi.org/10.1039/C2JM31649H
5.) Deshpande, et al. Opp-dibenzoporphyrins as a light-harvester for dye-sensitized solar cells. Chemistry – An Asian Journal, 2012, vol. 7, # 11, p. 2662 – 2669,8. https://doi.org/10.1002/asia.201200507
6.) Prakash, et al. Facile synthesis of β-functionalized “push-pull” Zn(II) porphyrins for DSSC applications. Dyes and Pigments, 2017, vol. 147, p. 56 – 66. https://doi.org/10.1016/j.dyepig.2017.07.053