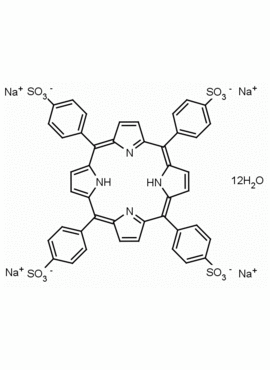
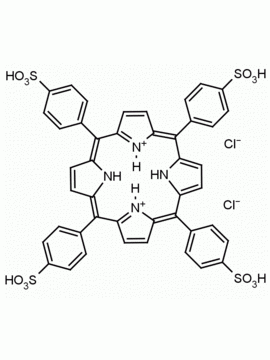
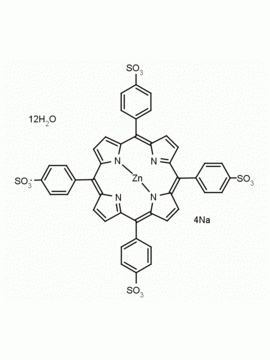
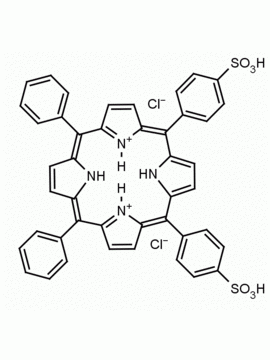
meso-Tetra(4-sulfonatophenyl) porphine tetrasodium salt dodecahydrate CAS: 39050-26-5 MDL: MFCD00151011
Molecular weight: 1239.099 g/mol
Molecular Formula: C44H50N4Na4O24S4
CAS Number: 39050-26-5
Storage: Store at room temperature, protect from light
Synonyms: 4,4′,4”,4”’-(5,10
Field of Interest: Water Soluble Porphyrins, Covalent Organic Frameworks, Photosensitizer,
Background: meso-Tetra(4-sulfonatophenyl) porphine tetrasodium salt dodecahydrate is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. meso-Tetra(4-sulfonatophenyl) porphine tetrasodium salt dodecahydrate was used as a precursor to synthesize several water soluble iron(II) nitroxyl porphyrin complexes.1 meso-Tetra(4-sulfonatophenyl) porphine tetrasodium salt dodecahydrate was used for the synthesis of water soluble ruthenium porphyrins as potential cytotoxic agents.2 meso-Tetra(4-sulfonatophenyl) porphine tetrasodium salt dodecahydrate was used in combination with a metallosurfactant for the self assembly of nano containers for drug delivery.3 meso-Tetra(4-sulfonatophenyl) porphine tetrasodium salt dodecahydrate was used for metal-free cellulose-based platforms for biomolecule fluorescence signal and SERS enhancement.4,5
SMILES: O=S(C1=CC=C(/C2=C3C=CC(/C(C4=CC=C(S(=O)([O-])=O)C=C4)=C5C=C/C(N/5)=C(C6=CC=C(S(=O)([O-])=O)C=C6)/C(C=C/7)=NC7=C(C8=CC=C(S(=O)([O-])=O)C=C8)/C9=CC=C2N9)=N3)C=C1)([O-])=O.[Na+].[Na+].[Na+].[Na+].O.O.O.O.O.O.O.O.O.O.O.O
References:
1.) Mazzeo, et al. Water-Soluble Nitroxyl Porphyrin Complexes FeIITPPSHNO and FeIITPPSNO– Obtained from Isolated FeIITPPSNO• J. Am. Chem. Soc. 2019, 141, 46, 18521–18530. https://doi.org/10.1021/jacs.9b09161
2.) Hartmann, M., Robert, A., Duarte, V. et al. Synthesis of water-soluble ruthenium porphyrins as DNA cleavers and potential cytotoxic agents. JBIC 2, 427–432 (1997). https://doi.org/10.1007/s007750050153
3.) Kashapov, et al. Supramolecular Self-Assembly of Porphyrin and Metallosurfactant as a Drug Nanocontainer Design. Nanomaterials 2022, 12(12), 1986; https://doi.org/10.3390/nano12121986
4.) Fularz, et al. Metal-Free Cellulose-Based Platforms for Biomolecule Fluorescence Signal Enhancement. ACS Sustainable Chem. Eng. 2022, 10, 1, 508–520. https://doi.org/10.1021/acssuschemeng.1c06995
5.) Fularz, et al. SERS Enhancement of Porphyrin-Type Molecules on Metal-Free Cellulose-Based Substrates. ACS Sustainable Chem. Eng. 2021, 9, 49, 16808–16819. https://doi.org/10.1021/acssuschemeng.1c06685
6.) Teplonogova, et al. Switchable Nanozyme Activity of Porphyrins Intercalated in Layered Gadolinium Hydroxide. Int. J. Mol. Sci. 2022, 23(23), 15373; https://doi.org/10.3390/ijms232315373