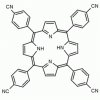
meso-Tetra(4-cyanophenyl) porphine CAS: 14609-51-9 MDL: MFCD00600964
Molecular weight: 714.77 g/mol
Molecular Formula: C48H26N8
CAS Number: 14609-51-9
Storage: Store at room temperature, protect from light.
Synonyms: 4,4′,4”,4”’-(5,10
Fields of Interest: Synthetic Porphyrins
Background: meso-Tetra(4-cyanophenyl) porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Lu, et al. Atomic Ni Anchored Covalent Triazine Framework as High Efficient Electrocatalyst for Carbon Dioxide Conversion. Advanced Functional Materials, 2019, vol. 29, # 10, art. no. 1806884. https://doi.org/10.1002/adfm.201806884
2.) Yi, et al. Cobalt single-atoms anchored on porphyrinic triazine-based frameworks as bifunctional electrocatalysts for oxygen reduction and hydrogen evolution reactions. Journal of Materials Chemistry A, 2019, vol. 7, # 3, p. 1252 – 1259. https://doi.org/10.1039/C8TA09490J
3.) Liu, et al. A Reversible Crystallinity-Preserving Phase Transition in Metal-Organic Frameworks: Discovery, Mechanistic Studies, and Potential Applications. Journal of the American Chemical Society, 2015, vol. 137, # 24, p. 7740 – 7746. https://doi.org/10.1021/jacs.5b02999
4.) Wang, et al. A stable metal cluster-metalloporphyrin MOF with high capacity for cationic dye removal. Journal of Materials Chemistry A, 2018, vol. 6, # 36, p. 17698 – 17705. https://pubs.rsc.org/en/content/articlelanding/2018/TA/C8TA06249H
5.) Cao, et al. Highly Selective Tandem Electroreduction of CO2 to Ethylene over Atomically Isolated Nickel–Nitrogen Site/Copper Nanoparticle Catalysts. Angewandte Chemie – International Edition, 2021, vol. 60, # 48, p. 25485 – 25492. https://doi.org/10.1002/anie.202111136
6.) Wang, et al. An ultrafast responsive NO2 gas sensor based on a hydrogen-bonded organic framework material. Chemical Communications, 2020, vol. 56, # 5, p. 703 – 706. https://doi.org/10.1039/C9CC09171H