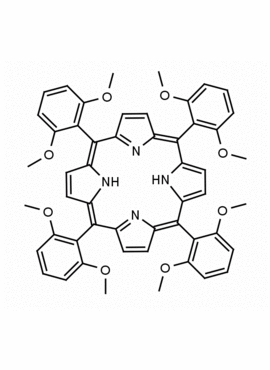
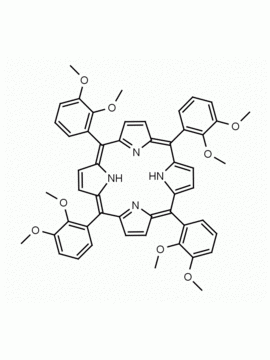
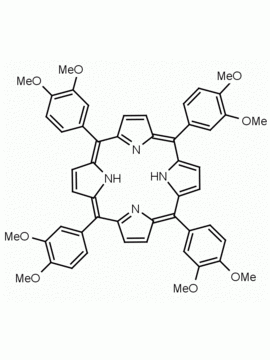
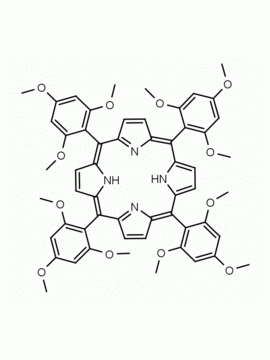
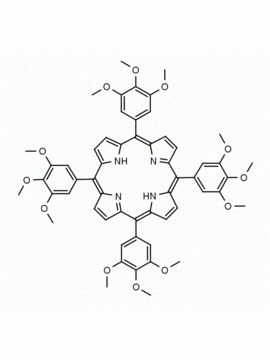
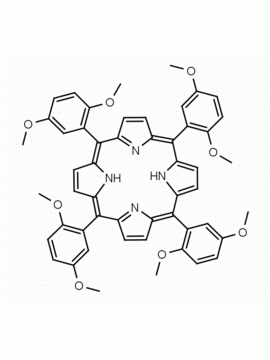
meso-Tetra(2,6-dimethoxyphenyl) porphine CAS: 118762-56-4 MDL: MFCD32668781
Molecular weight: 854.94 g/mol
Molecular Formula: C52H46N4O8
CAS Number: 118762-56-4
Storage: Store at room temperature, protect from light.
Synonyms: 1,3-Dimethoxy-2-[7,
Fields of Interest: Synthetic Porphyrins, Catalysis, Supramolecular Chemistry
Background: meso-Tetra(2,6-dimethoxyphenyl) porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Marianov, et al. Resolving deactivation pathways of Co porphyrin-based electrocatalysts for CO2 reduction in aqueous medium. ACS Catalysis, 2021, vol. 11, # 6, p. 3715 – 3729. https://doi.org/10.1021/acscatal.0c05092
2.) Bonnet, et al. Ligand Controls the Activity of Light-Driven Water Oxidation Catalyzed by Nickel(II) Porphyrin Complexes in Neutral Homogeneous Aqueous Solutions. Angewandte Chemie – International Edition, 2021, vol. 60, # 24, p. 13463 – 13469. https://doi.org/10.1002/anie.202103157
3.) Weng, et al. Electrochemical CO2 Reduction to Hydrocarbons on a Heterogeneous Molecular Cu Catalyst in Aqueous Solution. Journal of the American Chemical Society, 2016, vol. 138, # 26, p. 8076 – 8079. https://doi.org/10.1021/jacs.6b04746
4.) Bhyrappa, et al. Hydrogen-bonded porphyrinic solids: Supramolecular networks of octahydroxy porphyrins. Journal of the American Chemical Society, 1997, vol. 119, # 36, p. 8492 – 8502. https://doi.org/10.1021/ja971093w
5.) Huang, et al. Diastereoselective and enantioselective cyclopropanation of alkenes catalyzed by cobalt porphyrins. Journal of Organic Chemistry, 2003, vol. 68, # 21, p. 8179 – 8184. https://doi.org/10.1021/jo035088o
6.) Monnereau, et al. Porphyrin macrocyclic catalysts for the processive oxidation of polymer substrates. Journal of the American Chemical Society, 2010, vol. 132, # 5, p. 1529 – 1531. https://doi.org/10.1021/ja908524x