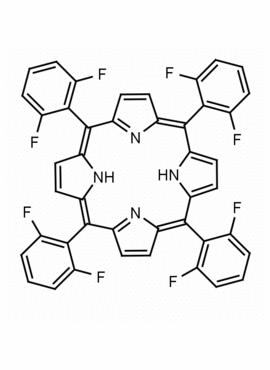
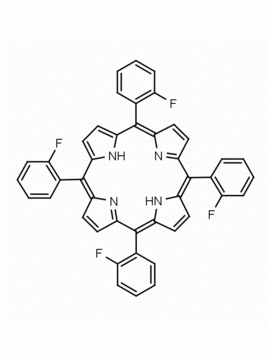
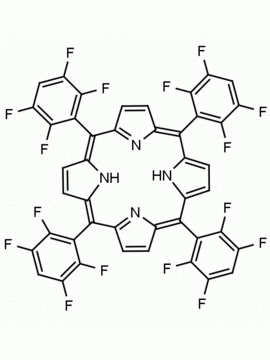
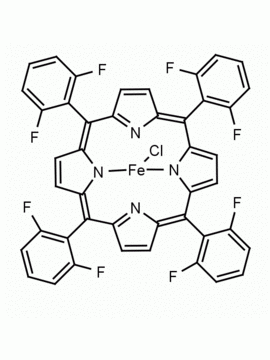
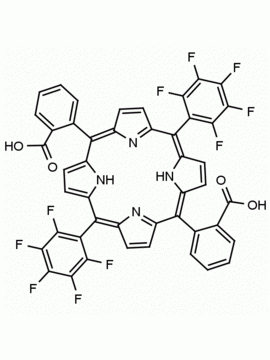
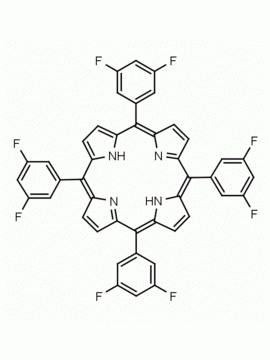
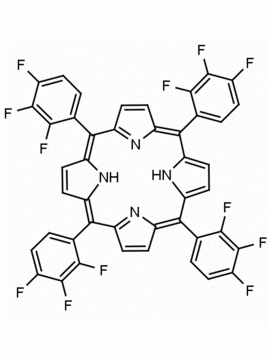
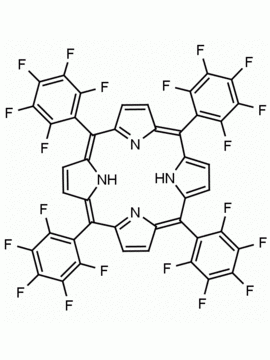
meso-Tetra(2,6-difluorophenyl) porphine CAS: 104322-39-6 MDL: MFCD00600965
Molecular weight: 758.66 g/mol
Molecular Formula: C44H22F8N4
CAS Number: 104322-39-6
Storage: Store at room temperature, protect from light
Synonyms: 21H,23H-Porphine, 5
Field of Interest: Synthetic Porphyrins,
Background: meso-Tetra(2,6-difluorophenyl) porphine is a synthetic porphyrin based specialty chemical.
References:
1.) Armaut, et al. Photodynamic therapy efficacy enhanced by dynamics: The role of charge transfer and photostability in the selection of photosensitizers. Chemistry – A European Journal, 2014, vol. 20, # 18, p. 5346 – 5357. https://doi.org/10.1002/chem.201304202
2.) Peraira, et al. An insight into solvent-free diimide porphyrin reduction: A versatile approach for meso-aryl hydroporphyrin synthesis. Green Chemistry, 2012, vol. 14, # 6, p. 1666 – 1672. https://doi.org/10.1039/C2GC35126A
3.) Yu, et al. Ruthenium-catalyzed oxidation of the porphyrin β,β-pyrrolic ring: A general and efficient approach to porpholactones. Advanced Synthesis and Catalysis, 2012, vol. 354, # 18, p. 3509 – 3516. https://doi.org/10.1002/adsc.201200720
4.) Cuesta-Aluja, et al. Halogenated meso-phenyl Mn(III) porphyrins as highly efficient catalysts for the synthesis of polycarbonates and cyclic carbonates using carbon dioxide and epoxides. Journal of Molecular Catalysis A: Chemical, 2016, vol. 423, p. 489 – 494. https://doi.org/10.1016/j.molcata.2015.10.025
5.) Henriques, et al. Biologically Inspired and Magnetically Recoverable Copper Porphyrinic Catalysts: A Greener Approach for Oxidation of Hydrocarbons with Molecular Oxygen. Advanced Functional Materials, 2016, vol. 26, # 19, p. 3359 – 3368. https://doi.org/10.1002/adfm.201505405
6.) Dabrowski, et al. New hybrid materials based on halogenated metalloporphyrins for enhanced visible light photocatalysis. RSC Advances, 2015, vol. 5, # 113, p. 93252 – 93261. https://doi.org/10.1039/C5RA19742B