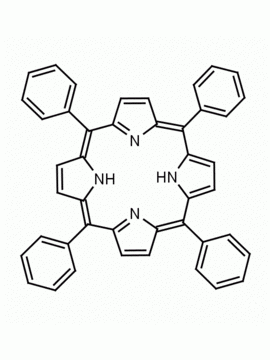
meso-Tetra(2,4,6-trimethylphenyl)porphine meso-Tetramesitylporphine CAS: 56396-12-4 MDL: MFCD01321200
Molecular weight: 783.1 g/mol
Molecular Formula: C56H54N4
CAS Number: 56396-12-4
Storage: Store at room temperature, protect from light
Synonyms: 56396-12-4, tetramesitylporphyrin, 5,10,15,20-tetrakis(2,4,6-trimethylphenyl)porphyrin, 5,10,15,20-Tetrakis(2,4,6-trimethylphenyl)-21H,23H-porphine, meso-tetra(2,4,6-trimethylphenyl)porphyrin, 2,7,12,17-tetrakis(2,4,6-trimethylphenyl), 21,22,23,24-tetraazapentacyclo[16.2.1.1(3),6.18,(1)(1).1(1)(3),(1)6]tetracosa, 1,3,5,7,9,11(23),12,14,16,18(21),19-undecaene, Tetrakistrimethylphenylporphin, SCHEMBL949016
Field of Interest: Synthetic Porphyrin, Catalyst, Radical Scavenger, Metal Organic Frameworks
Background: meso-Tetra(2,4,6-trimethylphenyl)porphine is a synthetic porphyrin based specialty chemical. Metallated derivatives of meso-Tetra(2,4,6-trimethylphenyl)porphine are catalytically active mimics of cytochrome P450.1 The Mn(III) metallated meso-Tetra(2,4,6-trimethylphenyl)porphine was found to be a catalyst for the epoxidation of alkenes.2 meso-Tetra(2,4,6-trimethylphenyl)porphine was used for the synthesis of high valent iron oxo complexes.3
References:
1.) Mansuy, A brief history of the contribution of metalloporphyrin models to cytochrome P450 chemistry and oxidation catalysis. Comptes Rendus Chimie Volume 10, Issues 4–5, April–May 2007, Pages 392-413. https://doi.org/10.1016/j.crci.2006.11.001
2.) Bagherzadeh, et al. Synthesis of dipyroromethanes in water and investigation of electronic and steric effects in efficiency of olefin epoxidation by sodium periodate catalyzed by manganese tetraaryl and trans disubstituted porphyrin complexes. Journal of Porphyrins and Phthalocyanines Vol. 23, No. 06, pp. 671-678 (2019). https://doi.org/10.1142/S108842461950038X
3.) Fujii, Electronic structure and reactivity of high-valent oxo iron porphyrins. Coordination Chemistry Reviews Volume 226, Issues 1–2, March 2002, Pages 51-60. https://doi.org/10.1016/S0010-8545(01)00441-6
4.) Cook, et al. Weakening the N–H Bonds of NH3 Ligands: Triple Hydrogen-Atom Abstraction to Form a Chromium(V) Nitride. Inorg. Chem. 2022, 61, 29, 11165–11172. https://doi.org/10.1021/acs.inorgchem.2c01115
5.) Kuijpers, et al. Cobalt-Porphyrin-Catalysed Intramolecular Ring-Closing C−H Amination of Aliphatic Azides: A Nitrene-Radical Approach to Saturated Heterocycles.Chemistry – A European Journal, 2017, vol. 23, # 33, p. 7945 – 7952. https://doi.org/10.1002/chem.201700358
6.) Groves, et al. Preparation and Characterization of an Iron(III) Porphyrin N-Oxide. Journal of the American Chemical Society, 1986, vol. 108, # 24, p. 7836 – 7837. https://doi.org/10.1021/ja00284a059
7.) Zhou, et al. Synthesis of β-mono-, tetra-, and octasubstituted sterically bulky porphyrins via Suzuki cross coupling. Journal of Organic Chemistry, 1996, vol. 61, # 11, p. 3590 – 3593. https://doi.org/10.1021/jo952205+
8.) Chen, et al. Two-photon absorbing block copolymer as a nanocarrier for porphyrin: Energy transfer and singlet oxygen generation in micellar aqueous solution. Journal of the American Chemical Society, 2007, vol. 129, # 23, p. 7220 – 7221. https://doi.org/10.1021/ja071057p
9.) Fukuzumi, et al. Mechanistic insights into hydride-transfer and electron-transfer reactions by a manganese(IV)-oxo porphyrin complex. Journal of the American Chemical Society, 2009, vol. 131, # 47, p. 17127 – 17134. https://doi.org/10.1021/ja9045235
10.) Ke, et al. Ytterbium(III) porpholactones: β-lactonization of porphyrin ligands enhances sensitization efficiency of lanthanide near-infrared luminescence. Chemistry – A European Journal, 2014, vol. 20, # 15, p. 4324 – 4333. https://doi.org/10.1002/chem.201303972
11.) Ramello, et al. Visible light induced oxygenation of alkenes with water sensitized by silicon-porphyrins with the second most earth-abundant element. Journal of Photochemistry and Photobiology A: Chemistry, 2015, vol. 313, p. 176 – 183. https://doi.org/10.1016/j.jphotochem.2015.07.016
12.) Groves, et al. Aliphatic Hydroxylation Catalyzed by Iron Porphyrin Complexes. Journal of the American Chemical Society, 1983, vol. 105, # 20, p. 6243 – 6248. https://doi.org/10.1021/ja00358a009