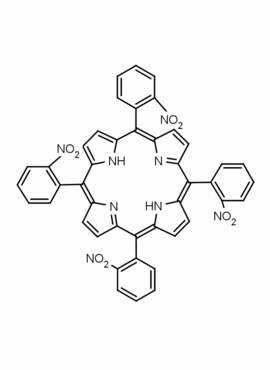
meso-Tetra(2-nitrophenyl) porphine CAS: 37116-82-8 MDL: MFCD03427530
Molecular weight: 794.73 g/mol
Molecular Formula: C44H26N8O8
CAS Number: 37116-82-8
Storage: Store at room temperature, protect from light
Synonyms: 21H,23H-Porphine, 5
Field of Interest: Synthetic Porphyrins,
Background: meso-Tetra(2-nitrophenyl) porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Collman, et al. Picket fence porphyrins. Synthetic models for oxygen binding hemoproteins. J. Am. Chem. Soc. 1975, 97, 6, 1427–1439. https://doi.org/10.1021/ja00839a026
2.) Apfel, et al. Homolytic versus Heterolytic Hydrogen Evolution Reaction Steered by a Steric Effect. Angewandte Chemie – International Edition, 2020, vol. 59, # 23, p. 8941 – 8946. https://doi.org/10.1002/anie.202002311
3.) Das, et al. Iron-Catalyzed Amination of Strong Aliphatic C(sp3)-H Bonds. Journal of the American Chemical Society, 2020, vol. 142, # 38, p. 16211 – 16217. https://doi.org/10.1021/jacs.0c07810
4.) Apfel, et al. Controlling Oxygen Reduction Selectivity through Steric Effects: Electrocatalytic Two-Electron and Four-Electron Oxygen Reduction with Cobalt Porphyrin Atropisomers. Angewandte Chemie – International Edition, 2021, vol. 60, # 23, p. 12742 – 12746. https://doi.org/10.1002/anie.202102523
5.) Nasrollahi, et al. Kinetics and mechanistic studies on the formation and reactivity of high valent MnO porphyrin species: Mono-: ortho or para -substituted porphyrins versus a di- ortho -substituted one. New Journal of Chemistry, 2018, vol. 42, # 3, p. 1806 – 1815. https://doi.org/10.1039/C7NJ04233G
6.) Khvostichenko, et al. Simple heme dimers with strongly cooperative ligand binding. Angewandte Chemie – International Edition, 2007, vol. 46, # 44, p. 8368 – 8370. https://doi.org/10.1002/anie.200702120