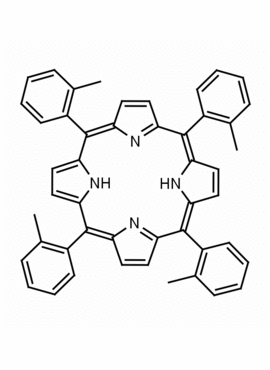
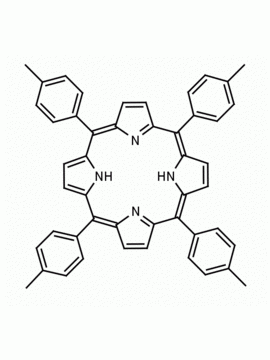
meso-Tetra(2-methylphenyl) porphine meso-Tetra(2-tolyl) porphine CAS: 37083-40-2 MDL: MFCD01074421
Molecular weight: 670.84 g/mol
Molecular Formula: C48H38N4
CAS Number: 37083-40-2
Storage: Store at room temperature, protect from light
Synonyms: 21H,23H-Porphine, 5
Field of Interest: Synthetic Porphyrins, Catalysis
Background: meso-Tetra(2-methylphenyl) porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. meso-Tetra(2-methylphenyl) porphine is sold as a mixture of atropisomers.
References:
1.) Sukiennik, et al. Manganese(iii) porphyrin-catalyzed regioselective dual functionalization of C(sp3)-H bonds: the transformation of arylalkanes to 1,4-diketones. Chemical Communications, 2023, vol. 59, # 9, p. 1149 – 1152. https://doi.org/10.1039/D2CC06126K
2.) Nasrollahi, et al. Kinetics and mechanistic studies on the formation and reactivity of high valent MnO porphyrin species: Mono-: ortho or para -substituted porphyrins versus a di- ortho -substituted one. New Journal of Chemistry, 2018, vol. 42, # 3, p. 1806 – 1815. https://pubs.rsc.org/en/content/articlelanding/2018/NJ/C7NJ04233G
3.) Khazaei, et al. Computational and experimental insights into the oxidative stability of iron porphyrins: A mono-ortho-substituted iron porphyrin with unusually high oxidative stability. Journal of Physical Organic Chemistry, 2018, vol. 31, # 11, art. no. E3869. https://onlinelibrary.wiley.com/doi/10.1002/poc.3869
4.) Tu, et al. Intriguing electrochemical behavior of free base porphyrins: Effect of porphyrin- meso -phenyl interaction controlled by position of substituents on meso -phenyls. Journal of Physical Chemistry A, 2012, vol. 116, # 6, p. 1632 – 1637. https://doi.org/10.1021/jp209555k
5.) Mojarrad, et al. Photocatalytic Activity of the Molecular Complexes of meso-Tetraarylporphyrins with Lewis Acids for the Oxidation of Olefins: Significant Effects of Lewis Acids and meso Substituents. European Journal of Inorganic Chemistry, 2017, vol. 2017, # 21, p. 2854 – 2862. https://doi.org/10.1002/ejic.201700264
6.) Shen, et al. Selective Solvent-Free and Additive-Free Oxidation of Primary Benzylic C–H Bonds with O2 Catalyzed by the Combination of Metalloporphyrin with N-Hydroxyphthalimide. Catalysis Letters, 2020, vol. 150, # 11, p. 3096 – 3111. https://doi.org/10.1007/s10562-020-03214-y