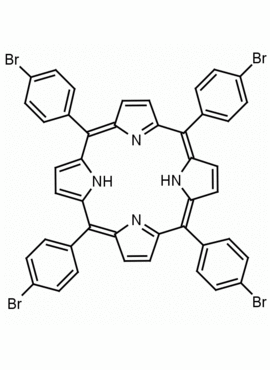
meso-Tetra (p-bromophenyl) porphine 5,10,15,20-Tetrakis(4-bromophenyl)-21H,23H-porphine CAS: 29162-73-0 MDL: MFCD02114459
Molecular weight: 930.32 g/mol
Molecular Formula: C44H26Br4N4
CAS Number: 29162-73-0
Storage: Store at room temperature, protect from light
Synonyms: 21H,23H-Porphine, 5
Field of Interest: Synthetic Porphyrins, Suzuki Coupling, Covalent Organic Frameworks COFs
Background: meso-Tetra (p-bromophenyl) porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Chen, et al. CMPs as Scaffolds for Constructing Porous Catalytic Frameworks: A Built-in Heterogeneous Catalyst with High Activity and Selectivity Based on Nanoporous Metalloporphyrin Polymers. J. Am. Chem. Soc. 2010, 132, 26, 9138–9143. https://doi.org/10.1021/ja1028556
2.) Lv, et al. A Base-Resistant Metalloporphyrin Metal–Organic Framework for C–H Bond Halogenation. J. Am. Chem. Soc. 2017, 139, 1, 211–217. https://doi.org/10.1021/jacs.6b09463
3.) Liang, et al. Porphyrin-based frameworks for oxygen electrocatalysis and catalytic reduction of carbon dioxide. Chem. Soc. Rev., 2021,50, 2540-2581. DOIhttps://doi.org/10.1039/D0CS01482F
4.) Wang, et al. A porous covalent porphyrin framework with exceptional uptake capacity of saturated hydrocarbons for oil spill cleanup. Chem. Commun., 2013,49, 1533-1535. https://doi.org/10.1039/C2CC38067F
5.) Yuan, et al. Effective and Selective Catalysts for Cinnamaldehyde Hydrogenation: Hydrophobic Hybrids of Metal–Organic Frameworks, Metal Nanoparticles, and Micro- and Mesoporous Polymers. Angewandte Chemie – International Edition, 2018, vol. 57, # 20, p. 5708 – 5713. https://doi.org/10.1002/anie.201801289
6.) Biswal, et al. Nonlinear Optical Switching in Regioregular Porphyrin Covalent Organic Frameworks. Angewandte Chemie – International Edition, 2019, vol. 58, # 21, p. 6896 – 6900. https://doi.org/10.1002/anie.201814412
7.) Lu, et al. Synthesis of a conjugated porous Co(ii) porphyrinylene–ethynylene framework through alkyne metathesis and its catalytic activity study. J. Mater. Chem. A, 2015,3, 4954-4959. https://doi.org/10.1039/C4TA06231K
8.) Liu, et al. Synthesis and photovoltaic properties of polythiophene stars with porphyrin core. Journal of Materials Chemistry, 2010, vol. 20, # 6, p. 1140 – 1146. https://doi.org/10.1039/B916935K
9.) Gao, et al. General and Efficient Synthesis of Arylamino- and Alkylamino-Substituted Diphenylporphyrins and Tetraphenylporphyrins via Palladium-Catalyzed Multiple Amination Reactions. J. Org. Chem. 2003, 68, 16, 6215–6221. https://doi.org/10.1021/jo034576t
10.) Eder, et al. A ruthenium porphyrin-based porous organic polymer for the hydrosilylative reduction of CO2 to formate. Chemical Communications, 2019, vol. 55, # 50, p. 7195 – 7198. https://doi.org/10.1039/C9CC02273B