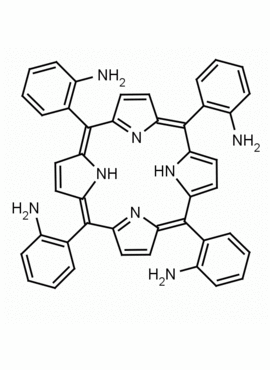
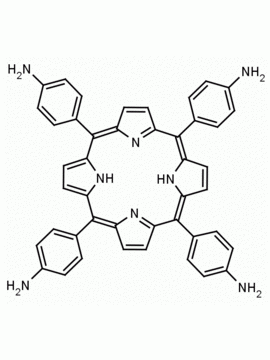
meso-Tetra (o-aminophenyl)porphine CAS: 52199-35-6 MDL: MFCD32267981
Molecular weight: 674.79 g/mol
Molecular Formula: C44H34N8
CAS Number: 52199-35-6
Storage: Store at room temperature, protect from light
Synonyms: 2,2′,2”,2”’-(5,10
Field of Interest: Synthetic Porphyrins, Amino Functionalized Porphyrins, Catalysis, Picket Fence Porphyrins
Background: meso-Tetra (o-aminophenyl)porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. meso-Tetra (o-aminophenyl)porphine is sold as a mixture of atropisomers. meso-Tetra (o-aminophenyl)porphine is of interest for constructing porphyrin based catalysts. If you are looking for a functionalized picket fence porphyrin, please see our custom porphyrins list.
References:
1.) Lv, et al. Controlling Oxygen Reduction Selectivity through Steric Effects: Electrocatalytic Two-Electron and Four-Electron Oxygen Reduction with Cobalt Porphyrin Atropisomers. Angewandte Chemie – International Edition, 2021, vol. 60, # 23, p. 12742 – 12746. https://doi.org/10.1002/anie.202102523
2.) Martin, et al. All Four Atropisomers of Iron Tetra(o–N,N,N-trimethylanilinium)porphyrin in Both the Ferric and Ferrous States. Inorg. Chem. 2021, 60, 7, 5240–5251. https://doi.org/10.1021/acs.inorgchem.1c00236
3.) Groves, et al. Catalytic asymmetric epoxidations with chiral iron porphyrins. J. Am. Chem. Soc. 1983, 105, 18, 5791–5796. https://doi.org/10.1021/ja00356a016
4.) Azcarate, et al. Through-Space Charge Interaction Substituent Effects in Molecular Catalysis Leading to the Design of the Most Efficient Catalyst of CO2-to-CO Electrochemical Conversion. J. Am. Chem. Soc. 2016, 138, 51, 16639–16644. https://doi.org/10.1021/jacs.6b07014
5.) Collman, et al. Picket fence porphyrins. Synthetic models for oxygen binding hemoproteins. J. Am. Chem. Soc. 1975, 97, 6, 1427–1439. https://doi.org/10.1021/ja00839a026
6.) Takeuchi, et al. Allosteric fluoride anion recognition by a doubly strapped porphyrin. Angewandte Chemie – International Edition, 2001, vol. 40, # 18, p. 3372 – 3376. https://doi.org/10.1002/1521-3773(20010917)40:18<3372::AID-ANIE3372>3.0.CO;2-1
7.) Kuroda, et al. Chiral Amino Acid Recognition by a Porphyrin-Based Artificial Receptor. J. Am. Chem. Soc. 1995, 117, 44, 10950–10958. https://doi.org/10.1021/ja00149a018
8.) Collman, et al. Synthesis and characterization of the “pocket” porphyrins. J. Am. Chem. Soc. 1983, 105, 10, 3038–3052. https://doi.org/10.1021/ja00348a018
9.) Taylor, et al. Porphyrin-based colorimetric sensing of perfluorooctanoic acid as proof of concept for perfluoroalkyl substance detection. Chem. Commun., 2021,57, 11649-11652. https://doi.org/10.1039/D1CC04903H
10.) Apfel, et al. Homolytic versus Heterolytic Hydrogen Evolution Reaction Steered by a Steric Effect. Angewandte Chemie – International Edition, 2020, vol. 59, # 23, p. 8941 – 8946. https://doi.org/10.1002/anie.202002311
11.) Das, et al. Iron-Catalyzed Amination of Strong Aliphatic C(sp3)–H Bonds J. Am. Chem. Soc. 2020, 142, 38, 16211–16217. https://doi.org/10.1021/jacs.0c07810
12.) Gotico, et al. Second-Sphere Biomimetic Multipoint Hydrogen-Bonding Patterns to Boost CO2 Reduction of Iron Porphyrins. Angewandte Chemie – International Edition, 2019, vol. 58, # 14, p. 4504 – 4509. https://doi.org/10.1002/anie.201814339