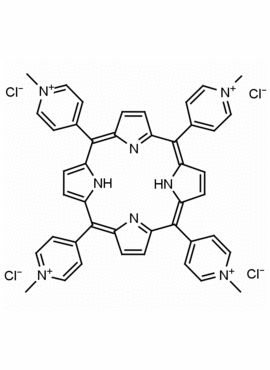
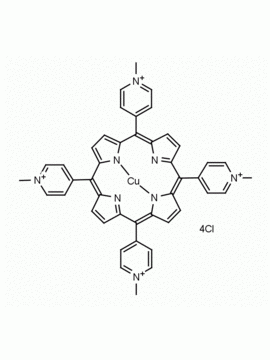
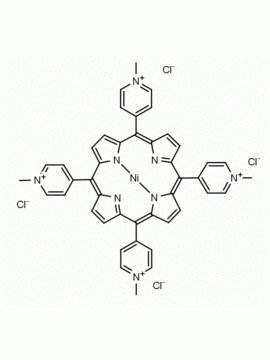
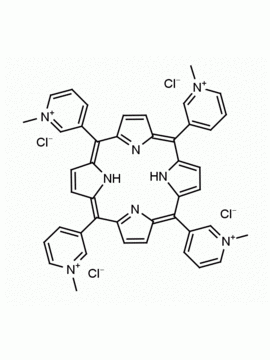
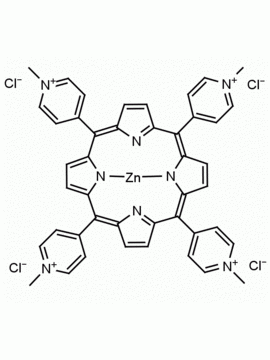
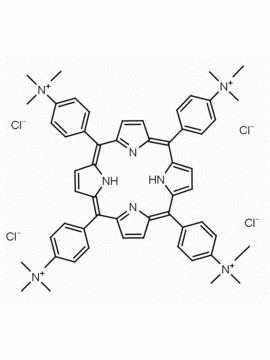
meso-Tetra (N-methyl-4-pyridyl) porphine tetrachloride CAS: 92739-63-4 MDL: MFCD09039048
Molecular weight: 820.638 g/mol
Molecular Formula: C44H38Cl4N8
CAS Number: 92739-63-4
Storage: Store at room temperature, protect from light
Synonyms: 4,4′,4”,4”’-(5,10
Field of Interest: Water Soluble Porphyrins, Sensors, Photosensitizer, Light Harvesting, G-Quadruplex
Background: meso-Tetra (N-methyl-4-pyridyl) porphine tetrachloride is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. meso-Tetra (N-methyl-4-pyridyl) porphine tetrachloride was used as a sensor in aqueous solutions for Hg+2, Pb+2, Cd+2 and Cu+2.1 meso-Tetra (N-methyl-4-pyridyl) porphine tetrachloride was used as a photosensitizer for the degradation of polyaromatic hydrocarbons.2 The energy transfer between meso-Tetra (N-methyl-4-pyridyl) porphine and clay nano sheets was studied.3 meso-tetra (N-methyl-4-pyridyl) porphine tetrachloride modulates αS aggregation in vitro.4
References:
1.) Zamadar, et al. Water Soluble Cationic Porphyrin Sensor for Detection of Hg2+, Pb2+, Cd2+, and Cu2+ Journal of Sensors. Volume 2016 Article ID 1905454
2.) Herschmann, et al. Effect of Toxic Metal Ions on Photosensitized Singlet Oxygen Generation for Photodegradation of Polyaromatic Hydrocarbon Derivatives and Inactivation of Escherichia coli. Photochemistry and Photobiology. Volume 95, Issue 3, May/June 2019,Pages 823-832. https://doi.org/10.1111/php.13050
3.) Ohtani, et al. Energy Transfer among Three Dye Components in a Nanosheet–Dye Complex: An Approach To Evaluating the Performance of a Light-Harvesting System. J. Phys. Chem. C 2017, 121, 4, 2052–2058. https://doi.org/10.1021/acs.jpcc.6b10372
4.) González, N., Gentile, I., Garro, H.A. et al. Metal coordination and peripheral substitution modulate the activity of cyclic tetrapyrroles on αS aggregation: a structural and cell-based study. J Biol Inorg Chem 24, 1269–1278 (2019). https://doi.org/10.1007/s00775-019-01711-z
5.) Khairutdinov, et al. Photophysical Deactivation Dynamics of Excited Porphyrin Molecules Adsorbed onto Dihexadecyl Phosphate Vesicles. J. Phys. Chem. B 1999, 103, 18, 3682–3686 https://doi.org/10.1021/jp984701i
6.) Adinehnia, et al. Predicting the Size Distribution in Crystallization of TSPP:TMPyP Binary Porphyrin Nanostructures in a Batch Desupersaturation Experiment. Cryst. Growth Des. 2014, 14, 12, 6599–6606. https://doi.org/10.1021/cg501506s
7.) Hamer, et al. Nanoparticles as template for porphyrin nanostructure growth. Journal of Porphyrins and Phthalocyanines. Vol. 23, No. 04n05, pp. 526-533 (2019). https://doi.org/10.1142/S1088424619500469
8.) Aly, et al. Molecular-structure Control of Ultrafast Electron Injection at Cationic Porphyrin–CdTe Quantum Dot Interfaces. J. Phys. Chem. Lett. 2015, 6, 5, 791–795. https://doi.org/10.1021/acs.jpclett.5b00235
9.) Alonso, et al. A rapid and sensitive high-throughput screening method to identify compounds targeting protein–nucleic acids interactions. Nucleic Acids Research, Volume 43, Issue 8, 30 April 2015, Page e52. https://doi.org/10.1093/nar/gkv069
10.) Almohammed, et al. Electric Field-Induced Chemical Surface-Enhanced Raman Spectroscopy Enhancement from Aligned Peptide Nanotube–Graphene Oxide Templates for Universal Trace Detection of Biomolecules. J. Phys. Chem. Lett. 2019, 10, 8, 1878–1887. https://doi.org/10.1021/acs.jpclett.9b00436
11.) Xing, et al. An Injectable Self-Assembling Collagen–Gold Hybrid Hydrogel for Combinatorial Antitumor Photothermal/Photodynamic Therapy. Volume 28, Issue 19, May 18, 2016, Pages 3669-3676. https://doi.org/10.1002/adma.201600284
12.) Phan, A., Kuryavyi, V., Gaw, H. et al. Small-molecule interaction with a five-guanine-tract G-quadruplex structure from the human MYC promoter. Nat Chem Biol 1, 167–173 (2005). https://doi.org/10.1038/nchembio723