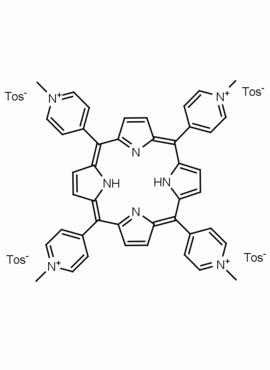
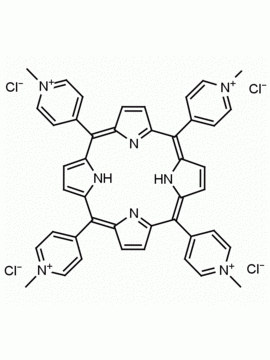
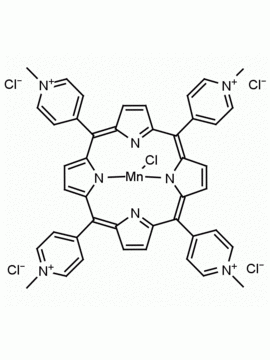
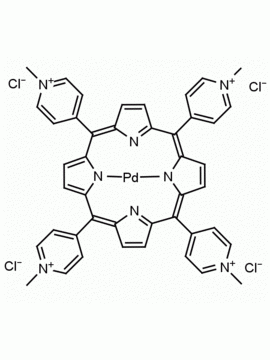
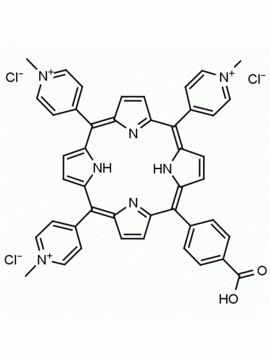
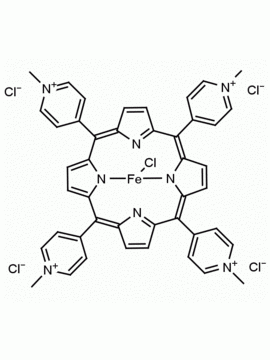
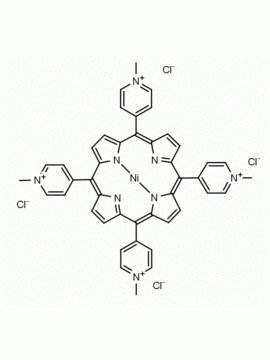
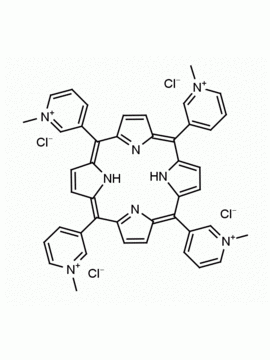
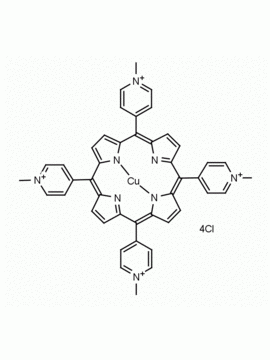
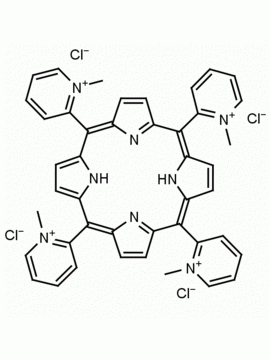
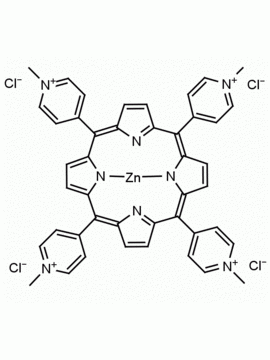
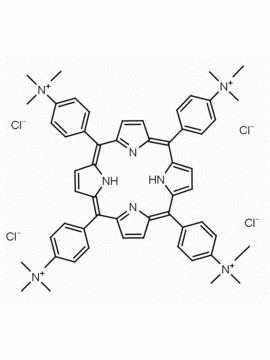
meso-Tetra (N-methyl-4-pyridyl) porphine tetra tosylate 5,10,15,20-Tetrakis (1-methyl-4-pyridinio) porphine tetra (p-toluenesulfonate) CAS: 36951-72-1 MDL: MFCD00013468
Molecular weight: 1363.601 g/mol
Molecular Formula: C72H66N8O12S4
CAS Number: 36951-72-1
Storage: Store at room temperature, protect from light
Synonyms: 4,4′,4”,4”’-(5,10,15,20-Porp
Field of Interest: Water Soluble Porphyrins, Photosensitizer, G-Quadruplex DNA
Background: meso-Tetra (N-methyl-4-pyridyl) porphine tetra tosylate is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. meso-Tetra (N-methyl-4-pyridyl) porphine tetra tosylate has been used as a photosensitizer for photodynamic therapy.1 meso-Tetra (N-methyl-4-pyridyl) porphine tetra tosylate was used for the cellular delivery of oligonucleotides.2 meso-Tetra (N-methyl-4-pyridyl) porphine tetra tosylate has been investigated for photodynamic antimicrobial chemotherapy.3
References:
1.) Abdelghany, et al. Enhanced Antitumor Activity of the Photosensitizer meso-Tetra(N-methyl-4-pyridyl) Porphine Tetra Tosylate through Encapsulation in Antibody-Targeted Chitosan/Alginate Nanoparticles. Biomacromolecules 2013, 14, 2, 302–310. https://doi.org/10.1021/bm301858a
2.) Flynn, et al. Water-Soluble, Meso-Substituted Cationic Porphyrins—a Family of Compounds for Cellular Delivery of Oligonucleotides. BioTechniques 26:736-746 (April 1999)
3.) Cassidy, et al. Drug delivery strategies for photodynamic antimicrobial chemotherapy: From benchtop to clinical practice. Journal of Photochemistry and Photobiology B: Biology. Volume 95, Issue 2, 4 May 2009, Pages 71-80. https://doi.org/10.1016/j.jphotobiol.2009.01.005
4.) Frederiksen, et al. Two-Photon Photosensitized Production of Singlet Oxygen in Water. J. Am. Chem. Soc. 2005, 127, 1, 255–269. https://doi.org/10.1021/ja0452020
5.) Pasternack, et al. Molecular complexes of nucleosides and nucleotides with a monomeric cationic porphyrin and some of its metal derivatives. J. Am. Chem. Soc. 1985, 107, 26, 8179–8186. https://doi.org/10.1021/ja00312a061
6.) Lang, et al. Layer-by-Layer Assembly of DNA Films and Their Interactions with Dyes. J. Phys. Chem. B 1999, 103, 51, 11393–11397. https://doi.org/10.1021/jp9915073
7.) Yamashita, et al. Stabilization of guanine quadruplex DNA by the binding of porphyrins with cationic side arms. Bioorganic & Medicinal Chemistry. Volume 13, Issue 7, 1 April 2005, Pages 2423-2430. https://doi.org/10.1016/j.bmc.2005.01.041
8.) Marchand, et al. Ligand-Induced Conformational Changes with Cation Ejection upon Binding to Human Telomeric DNA G-Quadruplexes. J. Am. Chem. Soc. 2015, 137, 2, 750–756. https://doi.org/10.1021/ja5099403
9.) Kubat, et al. Interaction of novel cationic meso-tetraphenylporphyrins in the ground and excited states with DNA and nucleotides. J. Chem. Soc., Perkin Trans. 1, 2000, 933-941. https://doi.org/10.1039/A909466K
10.) Zhang, et al. Stepwise Transformation of the Molecular Building Blocks in a Porphyrin-Encapsulating Metal–Organic Material. J. Am. Chem. Soc. 2013, 135, 16, 5982–5985. https://doi.org/10.1021/ja4015666
11.) Donnelly, et al. Microneedle Arrays Permit Enhanced Intradermal Delivery of a Preformed Photosensitizer. Photochemistry and Photobiology. Volume 85, Issue 1. January/February 2009, Pages 195-204. https://doi.org/10.1111/j.1751-1097.2008.00417.x
12.) Ikeda, et al. Photoelectrochemical sensor with porphyrin-deposited electrodes for determination of nucleotides in water. Organic Letters, 2009, vol. 11, # 5, p. 1163 – 1166. https://doi.org/10.1021/ol900037q